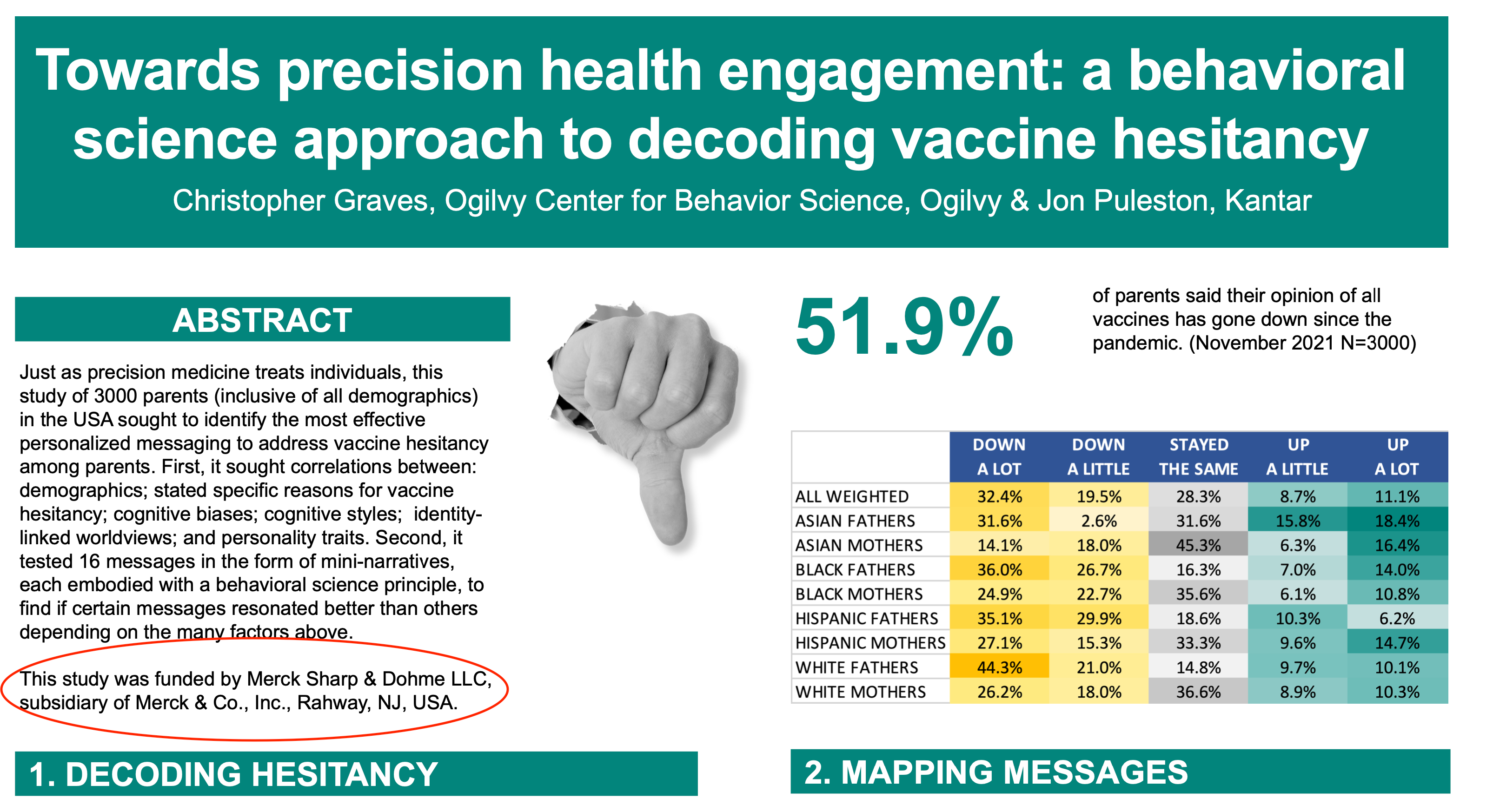
Very different from Covid, if there’s a known preventative measure for a disease the insurer has every right to decide to hedge their risk and drop their insured if that person refuses the treatment... Let the market work
Originally Posted by 1blackman1
So now comes the market to work. Appears the market and it's associated marketing didn't pan out after all. The stock price tumbled. It's now time for a change - back to the past even.
No I'm not talking about Bud Light. We're talking about vaccines, technically a gene therapy in this case. Turns out the plan is changed. It wasn't paying out. They went from a possible 70% take rate for the 2 shots of an experimental use authorization (EUA), which shielded all parties from liability, to mandatory requirement for a non-informed consent, actually first ever, mRNA gene therapy. However, the booster after booster take rate dropped to below 17%. Another loss for the marketing teams.
Time to change horses. Of course, many may have missed this Okey-Doke shuffle by way of vociferous distractions in the Lamb
SCREAM media. Dunno, war in the grifting and money laundering capital of the world or indictment of a former Presided on expired statute of limitations misdemeanors. What ever.
Staff Edit - Image Removed - Biomed1
FDA simplifies COVID vaccine schedules, withdraws authorization for older COVID-19 vaccines targeting virus' original strain
Vaccine uptake has slowed to a crawl in the U.S...
The Food and Drug Administration authorized a second omicron-targeted bivalent COVID-19 booster vaccine for people over 65 or who are immunocompromised, the agency announced Tuesday.
The agency also said any adult who has not yet been vaccinated will get one dose of an updated bivalent shot, rather than starting with the original vaccine and receiving the bivalent shot as a booster. The FDA is withdrawing authorization for those older COVID-19 vaccines targeting the original strain of the virus.
It's part of an overall move to simplify the COVID-19 vaccination process, the agency said in a statement. United States regulators are shifting towards a flu shot-like model for COVID-19 vaccines, where people get a single shot every year that's updated annually to match the virus strain predicted to be in circulation.
"At this stage of the pandemic, data support simplifying the use of the authorized mRNA bivalent COVID-19 vaccines and the agency believes that this approach will help encourage future vaccination," Peter Marks, director of the FDA's Center for Biologics Evaluation and Research, said in a statement.
The original COVID-19 vaccine was matched against the first strain of the virus first identified in Wuhan, China. That strain of the virus is no longer circulating — the omicron variant and its sub variants are the predominant strains throughout the world. The bivalent shots target both that original strain and the omicron variant. They were first authorized as booster doses in fall 2022 and research shows they offer stronger protection against infection and serious illness from omicron than the original shot...
..."Simple and clear messaging is critical when it comes to ensuring vaccine uptake," he said. "Moving to a model that resembles the yearly flu shot campaign will help focus messaging and help increase population immunity ahead of a potential fall surge."
FDA advisors will meet in June to discuss updating the vaccine composition for fall 2023...
So there you have it -- an annual flu shot (gene therapy) that they will do their best guess on in advance - just like the do with the other flu. Rumor also has it that they have reduced the percentage of active ingredient by up to 75%, but I've not verified that.
One might wonder what the efficacy of previous flu vaccines has been. About 43% through the years according to the CDC. The same CDC that just made the above changes BTW.
According to the CDC, aka serial liars, typical flu vaccines run around 36%-60% effective. Effective for what is murky, i.e. effective from preventing infection or hospitalizations.
Anyway here is their chart - notice the interesting annotation below it 
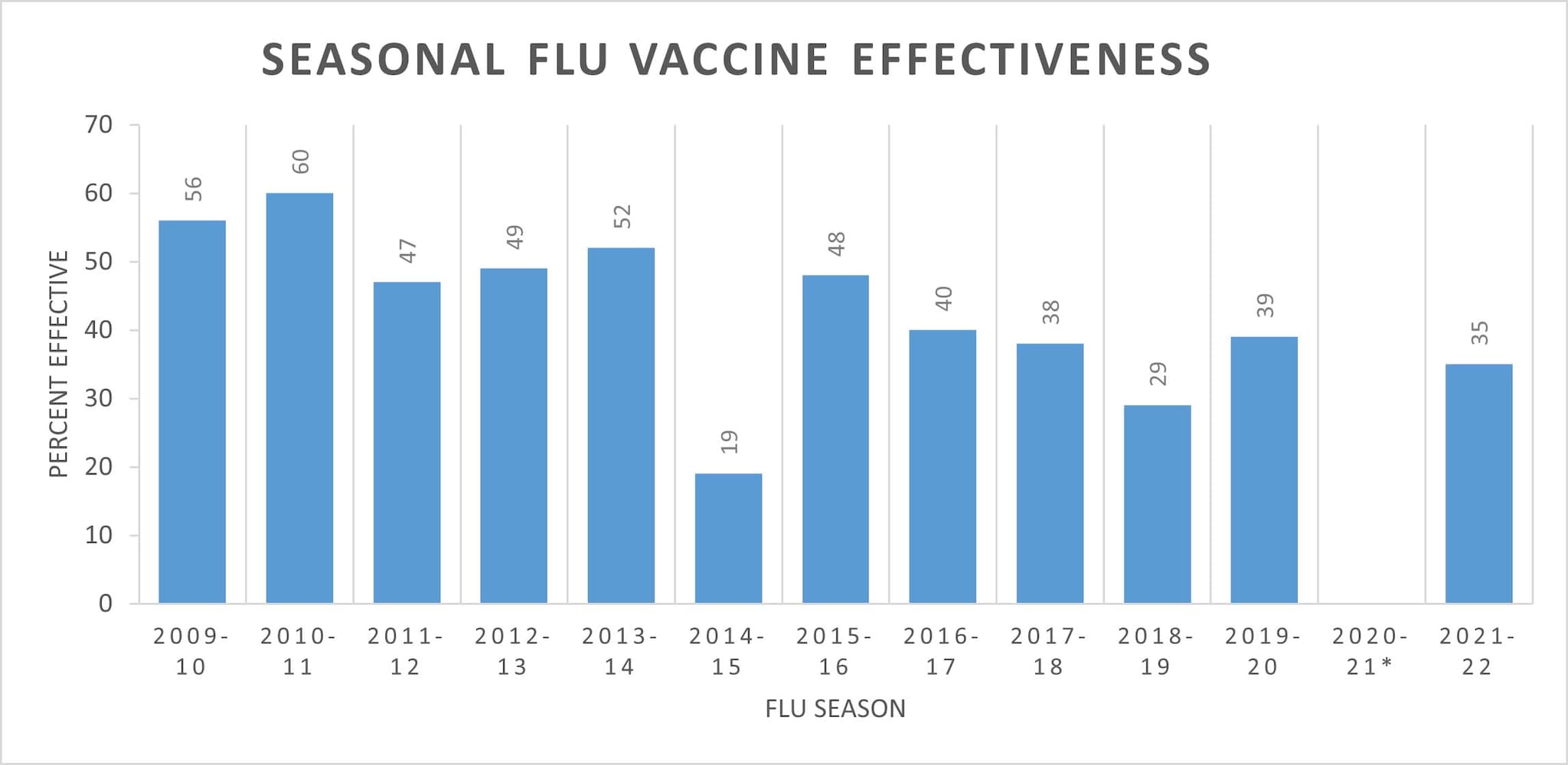
*2020-2021 flu vaccine effectiveness was not estimated due to low flu virus circulation during the 2020-2021 flu season.
Did ya catch that?!? No flu during the 'rona. Say wha??
FWIW: CDC Seasonal Flu Vaccine Effectiveness Studies
Originally Posted by Why_Yes_I_Do
Anyway, this new/old idea has been brewing most of 2023
An FDA committee votes to roll out a new COVID vaccination strategy
...A committee of advisers to the Food and Drug Administration voted unanimously on a proposal to simplify the nation's strategy for vaccinating people against COVID-19.
The recommendation is that future COVID-19 vaccines should be interchangeable: no matter whether you're getting your first dose or a booster, the vaccines would all have the same formulation targeting the same viral strain or strains, regardless of the manufacturer. The vote was unanimous: 21-0.
In addition, the committee considered (but didn't vote on) proposals to have an annual COVID vaccination schedule, much like the U.S. has for the flu. If this happens, most people would be advised to get just one shot every fall with a new vaccine that's probably been re-jiggered to try to match whatever variant is predicted to be spreading each winter. This would mean Americans would no longer need to keep track of how many shots they've already gotten or when.
The idea behind the revamp is to make vaccination less complicated and confusing. The ultimate goal would be to get more people vaccinated...
But this time, Socialism will actually work, but you can trust them this time Right Comrade?