HAS GALLO PROVEN THE ROLE OF HIV IN AIDS?
Eleni Papadopulos-Eleopulos, Valendar F. Turner, John M. Papadimitriou
ABSTRACT:
The evidence that Robert Gallo and his colleagues presented on 4th May 1984 regarding HTLV-III (HIV) isolation and the role of HIV in the pathogenesis of AIDS is critically analysed. It is concluded that the evidence does not constitute proof of the isolation of a retrovirus, that the virus is exogenous or that the virus is causally related to AIDS.
full paper: http://www.virusmyth.com/aids/hiv/epgallo.htm
.
- Mr. Bill
- 02-05-2012, 10:38 PM
- Mr. Bill
- 02-05-2012, 10:53 PM
Top Scientists Ask Medical Journal Science To Retract Original AIDS Papers
The international nonprofit scientific organization Rethinking AIDS gave its full support to 37 senior researchers, medical doctors and legal professionals who are requesting that the medical journal Science withdraw four seminal papers on HIV authored by Dr. Robert Gallo, papers widely touted as proof that HIV is the "probable cause of AIDS."
An online posting of the letter can be found here.
"With new findings that undermine the scientific integrity and veracity of Gallo's four papers, the entire basis of the theory that HIV causes AIDS may now be questioned," says Rethinking AIDS president David Crowe.
The letter to the journal comes at a time when the microbiology world is abuzz about Gallo's omission from the 2008 Nobel Prize in medicine for the discovery of HIV, contrary to an international agreement that the two teams should share credit. French scientists Drs. Luc Montagnier and Francoise Barré-Sinoussi are instead to be given the award, a decision that also implicitly questions the scientific integrity of Gallo's claim of the discovery. Montagnier, however, admitted on camera more than a decade ago that his experiments did not purify any virus.
The four papers (1, 2, 3, 4) were originally published on May 4, 1984, a few days after a press conference by Gallo announcing he had discovered the "probable cause of AIDS." Now, a British investigative journalist has shown that Gallo's claim was based on last-minute alterations to documents that make false claims about the results of his lab work and research experiments. The letter to Science sent by the 37 experts on Monday, Dec. 1, 2008, includes a copy of Gallo's handwritten changes to the article, a letter from an electron microscopy expert indicating that Gallo's samples did not contain any virus, and a letter from Gallo to a researcher verifying that HIV could not be purified directly from human materials.
The investigative conclusion prompting the letter to Science was made by journalist Janine Roberts, author of Fear of the Invisible, a book that examines the origin of several disease theories. "I was shocked when I read the original draft of the key scientific paper now widely cited as proving HIV causes AIDS," says Roberts. "Gallo's handwritten last-minute changes had reversed what the scientists in his lab had originally concluded. This demonstrates a stunning disregard for the scientific process and a very disturbing breach of public trust."
It is clear that the seminal research published on HIV contained unjustified claims and alterations. In 1993, governmental investigators determined Gallo had so poorly recorded his key and much-cited experiment that it was impossible to repeat and verify it.
In the early 1990s, several highly critical reports on the research underlying Gallo's papers were produced as a result of governmental inquiries working under the supervision of scientists nominated by the National Academy of Sciences and the Institute of Medicine. The Office of Research Integrity (ORI) of the U.S. Department of Health and Human Services concluded that the lead paper of the four was "fraught with false and erroneous statements" and that the "ORI believes that the careless and unacceptable keeping of research records . . . reflects irresponsible laboratory management that has permanently impaired the ability to retrace the important steps taken." Further, a Congressional Subcommittee on Oversight and Investigations produced a staff report on the papers, containing scathing criticisms of their integrity.
.
The international nonprofit scientific organization Rethinking AIDS gave its full support to 37 senior researchers, medical doctors and legal professionals who are requesting that the medical journal Science withdraw four seminal papers on HIV authored by Dr. Robert Gallo, papers widely touted as proof that HIV is the "probable cause of AIDS."
An online posting of the letter can be found here.
"With new findings that undermine the scientific integrity and veracity of Gallo's four papers, the entire basis of the theory that HIV causes AIDS may now be questioned," says Rethinking AIDS president David Crowe.
The letter to the journal comes at a time when the microbiology world is abuzz about Gallo's omission from the 2008 Nobel Prize in medicine for the discovery of HIV, contrary to an international agreement that the two teams should share credit. French scientists Drs. Luc Montagnier and Francoise Barré-Sinoussi are instead to be given the award, a decision that also implicitly questions the scientific integrity of Gallo's claim of the discovery. Montagnier, however, admitted on camera more than a decade ago that his experiments did not purify any virus.
The four papers (1, 2, 3, 4) were originally published on May 4, 1984, a few days after a press conference by Gallo announcing he had discovered the "probable cause of AIDS." Now, a British investigative journalist has shown that Gallo's claim was based on last-minute alterations to documents that make false claims about the results of his lab work and research experiments. The letter to Science sent by the 37 experts on Monday, Dec. 1, 2008, includes a copy of Gallo's handwritten changes to the article, a letter from an electron microscopy expert indicating that Gallo's samples did not contain any virus, and a letter from Gallo to a researcher verifying that HIV could not be purified directly from human materials.
The investigative conclusion prompting the letter to Science was made by journalist Janine Roberts, author of Fear of the Invisible, a book that examines the origin of several disease theories. "I was shocked when I read the original draft of the key scientific paper now widely cited as proving HIV causes AIDS," says Roberts. "Gallo's handwritten last-minute changes had reversed what the scientists in his lab had originally concluded. This demonstrates a stunning disregard for the scientific process and a very disturbing breach of public trust."
It is clear that the seminal research published on HIV contained unjustified claims and alterations. In 1993, governmental investigators determined Gallo had so poorly recorded his key and much-cited experiment that it was impossible to repeat and verify it.
In the early 1990s, several highly critical reports on the research underlying Gallo's papers were produced as a result of governmental inquiries working under the supervision of scientists nominated by the National Academy of Sciences and the Institute of Medicine. The Office of Research Integrity (ORI) of the U.S. Department of Health and Human Services concluded that the lead paper of the four was "fraught with false and erroneous statements" and that the "ORI believes that the careless and unacceptable keeping of research records . . . reflects irresponsible laboratory management that has permanently impaired the ability to retrace the important steps taken." Further, a Congressional Subcommittee on Oversight and Investigations produced a staff report on the papers, containing scathing criticisms of their integrity.
.
- Mr. Bill
- 02-05-2012, 11:21 PM
The Alchemy of Flow Cytometry
Since 2009, OMSJ has examined more than 100 criminal, civil and military cases related to testing, diagnosis and treatment of HIV and AIDS. In the majority of those cases, OMSJ found that clinicians who relied on high patient caseloads to generate revenue routinely use unreliable HIV tests to misdiagnose their patients.
by Nancy Banks, MD & Clark Baker
OMSJ has reported the widespread use of kickbacks used to bribe clinicians who unnecessarily prescribe deadly drugs to their healthy patients and found scarce evidence that those clinicians attempted to follow HHS or CDC guidelines to diagnose HIV. By the end of 2011, those kickbacks amounted to more than $750 million, which doesn’t include the millions paid to celebrities like Magic Johnson and Bono, whose propaganda campaign is now the focus of a multi-billion dollar international corruption scandal.
Weeks after testifying against her own patient, Bristol-Myers Squibb (BMS) sent Donna Sweet MD a $2.9 million grant. BMS is the maker of Sustiva (Atripla), which Dr. Sweet unnecessarily prescribed to her asymptomatic patient in 2010.
To justify the illegal marketing and distribution of toxins like AZT, Sustiva and Atripla, clinicians use tests that the FDA has never approved for diagnosing HIV in healthy patients.
DEFINING AIDS
The onset of the “AIDS pandemic” began with a tiny subculture of promiscuous homosexuals, many of whom attended circuit parties where they ingested meth, huffed carcinogens (amyl nitrate) and engaged in hours of unprotected anal sex (called barebacking). The drug-fueled colorectal trauma, injuries and comingling of fecal matter, semen and blood resulted in chronic infections and visits to doctors, who prescribed heavy doses of antibiotics that further compromised their patients’ immune function.
When some well-intended conservatives clumsily declared that the infections were related to “God’s intolerance of homosexuals,” it became politically suicidal to suggest that the self-destructive behavior of barebacking meth addicts had anything to do with the injuries incurred.

While gay activists pressured gullible celebrities into pushing the AIDS propaganda, scientists exploited the politics to secure funding. But despite the hysteria, Americans who didn’t abuse drugs or engage in anal intercourse didn’t acquire AIDS. Among the tiny number of heterosexuals who did, patients like Elizabeth Glazer and Ryan White succumbed only after taking fatal doses of the carcinogenic mutagen, Zidovudine (AZT). Their homicides were then used to promote the myth of heterosexual AIDS.
Despite the hysteria, actual AIDS cases were so rare that the CDC invented the Coolfont Estimate, which estimated the number of HIV infected persons in the U.S. They arrived at an estimate of between 1-1.5 million infections – a number extrapolated from the Kinsey Report, which estimated the number of homosexuals, IV drug users, hemophiliacs and heterosexuals who allegedly took part in homosexual acts in 1948.
Although the US population swelled from 228 million to more than 312 million since the alleged pandemic began, the CDC’s latest reports show that the initial estimates have remain unchanged at 1.2 million; a number that – if true – represents one-third of one percent of the total US population that (coincidentally) engages in self-destructive behavior.
To inflate these unremarkable numbers, the CDC changed the AIDS surveillance case definition “to include all HIV-infected persons who have less than 200 CD4+ T-lymphocytes/uL.” This change meant that – regardless of the incompetence of HIV tests – a lab that reports CD4 counts below 200 will, by definition, condemn healthy patients to a diagnosis of “full-blown AIDS” and a lifetime of deadly drugs that poisons patients.
continued...
.
Since 2009, OMSJ has examined more than 100 criminal, civil and military cases related to testing, diagnosis and treatment of HIV and AIDS. In the majority of those cases, OMSJ found that clinicians who relied on high patient caseloads to generate revenue routinely use unreliable HIV tests to misdiagnose their patients.
by Nancy Banks, MD & Clark Baker
OMSJ has reported the widespread use of kickbacks used to bribe clinicians who unnecessarily prescribe deadly drugs to their healthy patients and found scarce evidence that those clinicians attempted to follow HHS or CDC guidelines to diagnose HIV. By the end of 2011, those kickbacks amounted to more than $750 million, which doesn’t include the millions paid to celebrities like Magic Johnson and Bono, whose propaganda campaign is now the focus of a multi-billion dollar international corruption scandal.
Weeks after testifying against her own patient, Bristol-Myers Squibb (BMS) sent Donna Sweet MD a $2.9 million grant. BMS is the maker of Sustiva (Atripla), which Dr. Sweet unnecessarily prescribed to her asymptomatic patient in 2010.
To justify the illegal marketing and distribution of toxins like AZT, Sustiva and Atripla, clinicians use tests that the FDA has never approved for diagnosing HIV in healthy patients.
DEFINING AIDS
The onset of the “AIDS pandemic” began with a tiny subculture of promiscuous homosexuals, many of whom attended circuit parties where they ingested meth, huffed carcinogens (amyl nitrate) and engaged in hours of unprotected anal sex (called barebacking). The drug-fueled colorectal trauma, injuries and comingling of fecal matter, semen and blood resulted in chronic infections and visits to doctors, who prescribed heavy doses of antibiotics that further compromised their patients’ immune function.
When some well-intended conservatives clumsily declared that the infections were related to “God’s intolerance of homosexuals,” it became politically suicidal to suggest that the self-destructive behavior of barebacking meth addicts had anything to do with the injuries incurred.

While gay activists pressured gullible celebrities into pushing the AIDS propaganda, scientists exploited the politics to secure funding. But despite the hysteria, Americans who didn’t abuse drugs or engage in anal intercourse didn’t acquire AIDS. Among the tiny number of heterosexuals who did, patients like Elizabeth Glazer and Ryan White succumbed only after taking fatal doses of the carcinogenic mutagen, Zidovudine (AZT). Their homicides were then used to promote the myth of heterosexual AIDS.
Despite the hysteria, actual AIDS cases were so rare that the CDC invented the Coolfont Estimate, which estimated the number of HIV infected persons in the U.S. They arrived at an estimate of between 1-1.5 million infections – a number extrapolated from the Kinsey Report, which estimated the number of homosexuals, IV drug users, hemophiliacs and heterosexuals who allegedly took part in homosexual acts in 1948.
Although the US population swelled from 228 million to more than 312 million since the alleged pandemic began, the CDC’s latest reports show that the initial estimates have remain unchanged at 1.2 million; a number that – if true – represents one-third of one percent of the total US population that (coincidentally) engages in self-destructive behavior.
To inflate these unremarkable numbers, the CDC changed the AIDS surveillance case definition “to include all HIV-infected persons who have less than 200 CD4+ T-lymphocytes/uL.” This change meant that – regardless of the incompetence of HIV tests – a lab that reports CD4 counts below 200 will, by definition, condemn healthy patients to a diagnosis of “full-blown AIDS” and a lifetime of deadly drugs that poisons patients.
continued...
.
- Mr. Bill
- 02-05-2012, 11:29 PM
The Alchemy of Flow Cytometry
In one of OMSJ’s recent cases, a criminal defendant was charged with numerous counts of having exposed others to HIV. Although the HIV tests could be easily challenged, the fact that his lab reports showed CD4 counts as low as 32 and 7 suggested that he only had a few months to live – a fact refuted by his undeniable health.
For this reason, OMSJ began an investigation into the reliability and use of flow cytometry as it applies to HIV and AIDS.
FLOW CYTOMETRY
A careful reading of the medical and scientific journals establishes little correlation between CD4 cells and HIV. In one report, NIAID Director Anthony Fauci explained:
Several years later, Fauci added:
The aggregate CD4+T cell count is measured by a process known as flow cytometry – a measurement of characteristics of single cells suspended in a flowing saline stream moving through a beam of light. Many scientific procedures involve obtaining measurements as average values for the whole population, which differs from flow cytometric analysis measurements that are made on individual particles within the suspension.
Additionally, several parameters can be measured on tens of thousands of individual cells within a few minutes: relative size, relative granularity or internal complexity, and relative fluorescence intensity. Any suspended particle or cell from 0.2-150 micrometers in size is suitable for analysis. The noted characteristics are determined using an optical-to-electronic coupling system that records how the cell or particle scatters incident laser light and emits fluorescence.
However, flow cytometry has numerous intrinsically fatal shortcomings:
1. The fluidics system transports particles in a stream to the laser beam for interrogation.
2. The optics system consists of lasers to illuminate the particles in the sample stream and optical filters to direct the resulting light signals to the appropriate detectors.
3. The electronics system converts the detected light signals into electronic signals that can be processed by a computer. For some instruments equipped with a sorting feature, the electronics system is also capable of initiating sorting decisions to charge and deflect particles.
Flow cytometry evolved from the development of several fields: microscopy; dye chemistry; electronics; and computers. It was the fusion and advances of these diverse technologies that allowed for the evolution of flow cytometry.
Modern flow cytometry started at the Los Alamos National Laboratories in New Mexico and entered the marketplace by the mid-1970s. After scientists alleged in 1984 that HIV was killing CD4+T cells, researchers developed an advancement they called immunophenotyping.
Immunophenotyping is the analysis of heterogeneous populations of cells for the purpose of identifying the presence and proportions of the various populations of interest. Antibodies are used to identify cells by detecting specific antigens expressed by these cells, which are known as markers. These markers are usually functional membrane proteins involved in cell communication, adhesion, or metabolism. Immunophenotyping using flow cytometry has become the method of choice in identifying and sorting cells within complex populations and is used extensively in the diagnosis and management of AIDS. However, as noted above, the CD4+T cell population is not uniform and the nature of the Th1/Th2 shift has more to do with the development of AIDS than the aggregate decline of these cells.
continued...
.
In one of OMSJ’s recent cases, a criminal defendant was charged with numerous counts of having exposed others to HIV. Although the HIV tests could be easily challenged, the fact that his lab reports showed CD4 counts as low as 32 and 7 suggested that he only had a few months to live – a fact refuted by his undeniable health.
For this reason, OMSJ began an investigation into the reliability and use of flow cytometry as it applies to HIV and AIDS.
FLOW CYTOMETRY
A careful reading of the medical and scientific journals establishes little correlation between CD4 cells and HIV. In one report, NIAID Director Anthony Fauci explained:
“Although most studies necessarily focus on HIV infection of peripheral-blood mononuclear cells, the lymphocytes that are in the peripheral blood at any given time represent only about 2 percent of the total lymphocyte pool, most of which is in the lymphoid organs. Hence, in certain pathologic processes involving lymphoic cells, the peripheral blood may not accurately reflect the status of disease. Specific immune responses are generated predominantly in lymphoid organs rather than in the peripheral blood.”By analogy, the absence of policemen at a city park does not necessarily mean that the park is unsafe. The park may be safer than a disturbance where policemen are found in larger numbers. While the overall numbers of policemen won’t change in a city, their deployment depends upon when and where they are needed.
Several years later, Fauci added:
“…the primary mechanisms of CD4+T cell depletion in vivo remain unclear; there is no direct evidence that HIV is cytopathic in vivo, despite the fact that cytopathicity can be readily demonstrated in the artificial milieu of culture.”Some researchers have identified numerous subsets of CD4 cells, while others have found that low T-cell numbers are routinely found among life insurance applicants and African populations. Others have found viral load and flow cytometry equally dubious.
The aggregate CD4+T cell count is measured by a process known as flow cytometry – a measurement of characteristics of single cells suspended in a flowing saline stream moving through a beam of light. Many scientific procedures involve obtaining measurements as average values for the whole population, which differs from flow cytometric analysis measurements that are made on individual particles within the suspension.
Additionally, several parameters can be measured on tens of thousands of individual cells within a few minutes: relative size, relative granularity or internal complexity, and relative fluorescence intensity. Any suspended particle or cell from 0.2-150 micrometers in size is suitable for analysis. The noted characteristics are determined using an optical-to-electronic coupling system that records how the cell or particle scatters incident laser light and emits fluorescence.
However, flow cytometry has numerous intrinsically fatal shortcomings:
- While the observed cell stream is three dimensional, the scatter pattern that is generated on computer print-out of the cellular density stream is measured in two dimensions.
- The ability to identify live individual cells of a particular type from dead cells, clumps of cells and debris by a process known as gating is limited by the training and expertise of the observer-technician.
- There are no established standards for the technology of operators. Procedures vary between each technician and lab.
- All FDA-approved flow cytometry devices are based upon “predicate devices” technologies that were marketed before May 28, 1976.
- Researchers claim that the distinguishing characteristics of live individual cells from dead cells and debris can be accurately preserved following paraformaldehyde fixation (which kills all of the cells).
1. The fluidics system transports particles in a stream to the laser beam for interrogation.
2. The optics system consists of lasers to illuminate the particles in the sample stream and optical filters to direct the resulting light signals to the appropriate detectors.
3. The electronics system converts the detected light signals into electronic signals that can be processed by a computer. For some instruments equipped with a sorting feature, the electronics system is also capable of initiating sorting decisions to charge and deflect particles.
Flow cytometry evolved from the development of several fields: microscopy; dye chemistry; electronics; and computers. It was the fusion and advances of these diverse technologies that allowed for the evolution of flow cytometry.
Modern flow cytometry started at the Los Alamos National Laboratories in New Mexico and entered the marketplace by the mid-1970s. After scientists alleged in 1984 that HIV was killing CD4+T cells, researchers developed an advancement they called immunophenotyping.
Immunophenotyping is the analysis of heterogeneous populations of cells for the purpose of identifying the presence and proportions of the various populations of interest. Antibodies are used to identify cells by detecting specific antigens expressed by these cells, which are known as markers. These markers are usually functional membrane proteins involved in cell communication, adhesion, or metabolism. Immunophenotyping using flow cytometry has become the method of choice in identifying and sorting cells within complex populations and is used extensively in the diagnosis and management of AIDS. However, as noted above, the CD4+T cell population is not uniform and the nature of the Th1/Th2 shift has more to do with the development of AIDS than the aggregate decline of these cells.
continued...
.
- Mr. Bill
- 02-05-2012, 11:33 PM
The Alchemy of Flow Cytometry
FROM RESEARCH TO JUNK SCIENCE
In 1972, Congress established the Office of Technology Assessment (OTA) to serve the legislative branch as an independent source of information and analysis about complex scientific and technical issues. OTA construed health technology broadly, including “all elements of medical practice that are knowledge-based, including hardware (equipment and facilities) and software (knowledge skills)… the set of techniques, drugs, equipment, and procedures used by health care professionals in delivering medical care to individuals and the systems within which such care is delivered.”
By 1978, the OTA produced a shattering report on the state of scientific medicine, Assessing the Efficacy and Safety of Medical Technologies. The report stated:
The U.S. News and World Report issue of 23 November 1987 raised further questions about HIV tests:
In 1996, Congress disbanded the OTA, leading to the systematic deregulation of the various medical technology industries. The U.S. Supreme Court sealed the fate of future scientific transparency by ruling in favor or corporate interests in the Citizens United v. Federal Election Commission (2010).
The OTA’s demise closed a low-budget item that gave Americans too much information to make informed choices. It paved the way for the establishment of a medical and scientific knowledge monopoly that is now permeated by corruption and fraud (junk science).
As a result, the “AIDS industry” continues to use unproven technologies like flow cytometry to diagnose, alarm, and treat healthy people with toxic and expensive drugs that are designed to make them sick (see video).
Fundamental issues regarding the limitations of flow cytometry technology and the propriety of its use in the diagnosis of acquired immune deficiency have never been addressed:
Police officers also use RADAR to enforce basic speed laws. But like GC, officers do not rely upon RADAR devices to enforce laws. Instead, officers rely upon their training and expertise to estimate intoxication and velocities. Once they develop articulable facts that indicate impairment or an unsafe speed, they use GC and RADAR to confirm what their training, expertise and observations initially tell them.
Unlike GC and RADAR, flow cytometry manufacturers compensate for their inaccuracies by inventing proprietary algorithms to report spurious and unreliable cell counts. There is no reliable method for counting standardization for products and operators, and the substantial deviations in technical competency and quality control of test samples between labs render these tests wholly unreliable. Clinicians simply order blood draws and presume that lab results are accurate and meaningful. Clinicians who receive kickbacks from labs and drug companies have little incentive to question results of their asymptomatic patients.
This protocol is akin to policemen who stop 35 mph motorists because their RADAR device captured a 90mph reading. But while police agencies would train or terminate such derelict employees, this practice – when applied to biological testing and flow cytometry – is considered the “medical standard of care.”
Flow cytometry employs two techniques to count cells:
To assure the accuracy and reliability of CD4+T cell test results obtained within individual laboratories and to attempt to assure comparability of results between laboratories, the CDC established a list of standard methods for performing the test, as well as guidelines for quality control and quality assurance. The CDC’s recommendations for flow cytometry apply to laboratory safety, specimen collection, specimen transport, maintenance of specimen integrity, specimen processing, flow cytometer quality control, sample analyses, data analysis, data storage, data reporting and quality assurance.
As can be seen, there are multiple steps in this process, any of which that if violated can lead to a substantial alteration in the test results:
1. Blood collection: The type of vial, the time of day and the temperature at which the specimen is handled can all have an effect on test results. CD4+T cell counts are known to be higher in the afternoon than in the morning – a result of the response to the diurnal variation in steroid production from the adrenal gland.
2. Specimen transport: Was the specimen maintained at room temperature? If the specimen is too hot or too cold, cellular destruction might occur. This can be a major problem for transporting of the specimen outside of the collection facility.
3. Specimen Integrity: When the specimen was received, was it too hot or too cold? Was the blood hemolyzed or frozen? Are there visible clots? Has the specimen been received > 72 hours after collection? If so, the specimen must be rejected.
4. Specimen processing: The test should be run within 48 hours, but no later than 72 hours after drawing the blood. Procedures that must be followed:
5. Gently rocking blood for 5 minutes to ensure uniform sample
6. Pipetting accurate blood volumes; vortex sample tubes to mix the blood and reagents and break up cell aggregates
7. Quality and type of reagent used
8. Incubating tubes in dark during staining procedure
9. A lyse/no wash method which requires following manufacture directions (each manufactures has a different set of directions and a different counting algorithm)
10. An immediate capping and storing all stained samples in the dark under refrigeration until flow cytometric analysis is performed.
11. It is advised that the specimens be stored no more than 24 hours unless the laboratory can demonstrate that scatter and fluorescence patterns do not change for specimens stored for longer periods.
12. Machine calibration: Variations in absolute lymphocyte counts obtained by different automated cell counters exposes another problem. A review of four widely used automated counters indicate that analytic variability in the absolute lymphocyte counts due primarily to method variability, is significant and larger than the variability typically observed on inter-laboratory trials of relative CD4+T cell counts. These method biases cannot easily be reduced by calibration, since the cell classification algorithms are built in features of the various counters.
As can be seen, the process of flow cytometry using immunophenotyping requires not only a sophisticated level of technical skill, but a chain of delivery and processing events that is probably difficult to replicate from lab to lab, but also to substantiate. One study found errors of 18% and 35% of absolute CD4+T cell count, while another study found the inter-laboratory variability so significant that it led to conflicting treatment recommendations.
continued...
.
FROM RESEARCH TO JUNK SCIENCE
In 1972, Congress established the Office of Technology Assessment (OTA) to serve the legislative branch as an independent source of information and analysis about complex scientific and technical issues. OTA construed health technology broadly, including “all elements of medical practice that are knowledge-based, including hardware (equipment and facilities) and software (knowledge skills)… the set of techniques, drugs, equipment, and procedures used by health care professionals in delivering medical care to individuals and the systems within which such care is delivered.”
By 1978, the OTA produced a shattering report on the state of scientific medicine, Assessing the Efficacy and Safety of Medical Technologies. The report stated:
“Evidence indicates that many technologies are not adequately assessed before they enjoy widespread use… Many technologies which have been used extensively have later been shown to be of limited usefulness”…and ” … only 10 to 20 percent of all procedures currently used in medical practice have been shown to be efficacious by controlled trial.”The report implied that 80% to 90% of all routinely-performed procedures are unproven – a conclusion that implicates the technology of flow cytometry that uses immunophenotyping to identify antigen markers on various cell populations.
The U.S. News and World Report issue of 23 November 1987 raised further questions about HIV tests:
“With public health officials and politicians thrashing out who should be tested for HIV, the accuracy of the test itself has been ignored. A study last month by the Congressional Office of Technology Assessment found that HIV tests can be very inaccurate indeed. For groups at very low risk – people who do not use IV drugs or have sex with gay or bisexual men – 9 in 10 positive findings are called false positives, indicating infection where none exists.”OTA’s warning continues to be ignored by the medical and scientific communities and the politicians, agencies and regulators that enable them.
In 1996, Congress disbanded the OTA, leading to the systematic deregulation of the various medical technology industries. The U.S. Supreme Court sealed the fate of future scientific transparency by ruling in favor or corporate interests in the Citizens United v. Federal Election Commission (2010).
The OTA’s demise closed a low-budget item that gave Americans too much information to make informed choices. It paved the way for the establishment of a medical and scientific knowledge monopoly that is now permeated by corruption and fraud (junk science).
As a result, the “AIDS industry” continues to use unproven technologies like flow cytometry to diagnose, alarm, and treat healthy people with toxic and expensive drugs that are designed to make them sick (see video).
Fundamental issues regarding the limitations of flow cytometry technology and the propriety of its use in the diagnosis of acquired immune deficiency have never been addressed:
- Proofthat an exogenous virus uniquely attacks CD4+T cells and is cytopathic (see above discussion). Further, the relationship of the Th1/Th2 balance shift in the CD4+T cell population has been ignored.
- Reproducibility – samples drawn concurrently from one subject should deliver the same results in every machine and laboratory that receives those specimens.
Police officers also use RADAR to enforce basic speed laws. But like GC, officers do not rely upon RADAR devices to enforce laws. Instead, officers rely upon their training and expertise to estimate intoxication and velocities. Once they develop articulable facts that indicate impairment or an unsafe speed, they use GC and RADAR to confirm what their training, expertise and observations initially tell them.
Unlike GC and RADAR, flow cytometry manufacturers compensate for their inaccuracies by inventing proprietary algorithms to report spurious and unreliable cell counts. There is no reliable method for counting standardization for products and operators, and the substantial deviations in technical competency and quality control of test samples between labs render these tests wholly unreliable. Clinicians simply order blood draws and presume that lab results are accurate and meaningful. Clinicians who receive kickbacks from labs and drug companies have little incentive to question results of their asymptomatic patients.
This protocol is akin to policemen who stop 35 mph motorists because their RADAR device captured a 90mph reading. But while police agencies would train or terminate such derelict employees, this practice – when applied to biological testing and flow cytometry – is considered the “medical standard of care.”
Flow cytometry employs two techniques to count cells:
- Dual Platform Systems – One component determines cell concentrations, while the second determines the relative number of CD4 and CD8 cells. Unless these two components count a common parameter, dual platform systems cannot accurately correlate the results.
- Single Platform Systems – These platforms are especially designed to count the absolute numbers of antibody-labeled cells. These devices are equipped with multiple sample loader, programming facility and computer support, which removes the need for using two different machine to determine the concentration of CD4 and CD8 cells.
- Standardization – HHS, CDC, NIH and FDA have failed to produce meaningful guidelines for quality control, quality assurance or quality of test reagents.
To assure the accuracy and reliability of CD4+T cell test results obtained within individual laboratories and to attempt to assure comparability of results between laboratories, the CDC established a list of standard methods for performing the test, as well as guidelines for quality control and quality assurance. The CDC’s recommendations for flow cytometry apply to laboratory safety, specimen collection, specimen transport, maintenance of specimen integrity, specimen processing, flow cytometer quality control, sample analyses, data analysis, data storage, data reporting and quality assurance.
As can be seen, there are multiple steps in this process, any of which that if violated can lead to a substantial alteration in the test results:
1. Blood collection: The type of vial, the time of day and the temperature at which the specimen is handled can all have an effect on test results. CD4+T cell counts are known to be higher in the afternoon than in the morning – a result of the response to the diurnal variation in steroid production from the adrenal gland.
2. Specimen transport: Was the specimen maintained at room temperature? If the specimen is too hot or too cold, cellular destruction might occur. This can be a major problem for transporting of the specimen outside of the collection facility.
3. Specimen Integrity: When the specimen was received, was it too hot or too cold? Was the blood hemolyzed or frozen? Are there visible clots? Has the specimen been received > 72 hours after collection? If so, the specimen must be rejected.
4. Specimen processing: The test should be run within 48 hours, but no later than 72 hours after drawing the blood. Procedures that must be followed:
5. Gently rocking blood for 5 minutes to ensure uniform sample
6. Pipetting accurate blood volumes; vortex sample tubes to mix the blood and reagents and break up cell aggregates
7. Quality and type of reagent used
8. Incubating tubes in dark during staining procedure
9. A lyse/no wash method which requires following manufacture directions (each manufactures has a different set of directions and a different counting algorithm)
10. An immediate capping and storing all stained samples in the dark under refrigeration until flow cytometric analysis is performed.
11. It is advised that the specimens be stored no more than 24 hours unless the laboratory can demonstrate that scatter and fluorescence patterns do not change for specimens stored for longer periods.
12. Machine calibration: Variations in absolute lymphocyte counts obtained by different automated cell counters exposes another problem. A review of four widely used automated counters indicate that analytic variability in the absolute lymphocyte counts due primarily to method variability, is significant and larger than the variability typically observed on inter-laboratory trials of relative CD4+T cell counts. These method biases cannot easily be reduced by calibration, since the cell classification algorithms are built in features of the various counters.
As can be seen, the process of flow cytometry using immunophenotyping requires not only a sophisticated level of technical skill, but a chain of delivery and processing events that is probably difficult to replicate from lab to lab, but also to substantiate. One study found errors of 18% and 35% of absolute CD4+T cell count, while another study found the inter-laboratory variability so significant that it led to conflicting treatment recommendations.
continued...
.
- Mr. Bill
- 02-05-2012, 11:37 PM
The Alchemy of Flow Cytometry
PRODUCT RECALLS
Since 2004, the FDA has issued 66 recalls of flow cytometry products, devices, components and computer software.
The FDA has issued numerous warning letters to PointCare Technologies, a leading developer and producer of flow cytometry products. In its latest warning letter dated 14 June 2011, the FDA cited PointCare’s repeated and unresolved problems with their testing equipment, reagents and manufacturing facilities:
Of the 66 aforementioned recalls and warning letters, FDA complaints were issued for failures that typically result in low CD4 counts. For agencies that need low counts to claim high HIV infection rates e.g. revenues (and for clinicians who accept kickbacks and bribes from companies like Bristol-Myers Squibb [BMS] and Gilead Sciences), flow cytometry helps clinicians justify the unnecessary delivery of toxic HAART therapies to healthy patients.
Flow cytometry is especially helpful in places like Africa, where mining companies routinely dump toxins and heavy metals into waterways.
One company is Kilembe Mines Limited, which is trying to sell an operation that has polluted the environment and water supplies of southwestern Uganda since 1956. Because foreign investors are reluctant to assume liability that comes with the purchase of toxic waste dumps, clinics and medical devices that blame pollution-caused ailments on HIV offer significant advantages to prospective investors.
According to this report, the Uganda Catholic Medical Bureau (UCMB) has distributed drugs in this area for 30 years.
In an effort to “scale up access to antiretroviral therapy…” UCMB advised that “treatment procedures and the monitoring of clients are simplified so that lower cadres of health workers can be trained to carry out some of the simpler functions… (of HIV testing, diagnosis and treatment).”
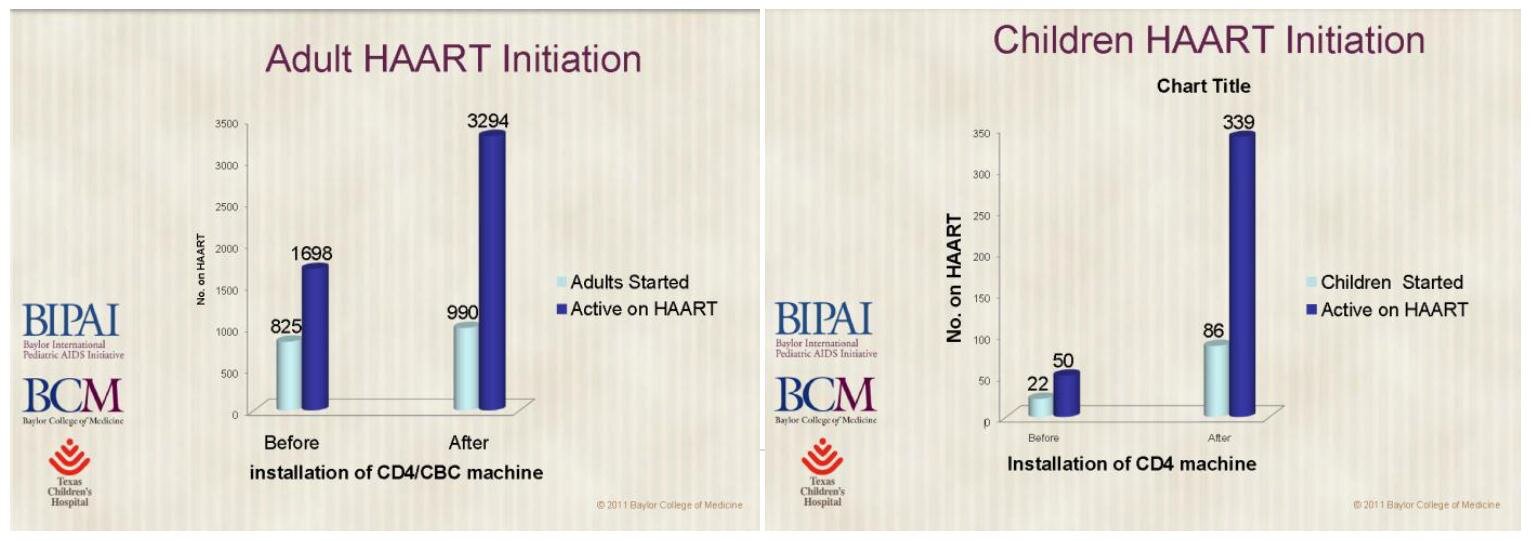
Gloria Kakuru of the Baylor University’s International Pediatric AIDS Initiative (BIPAI) explains how PointCare Now helps these “clinicians” diagnose HIV in the area surrounding the Kilembe mines – claiming that the device is superior because it justifies earlier initiation of HAART medication in adults and children:
These inflated misdiagnoses are then used by agencies like UNAIDS, the World Health Organization (WHO) and drug companies like Bristol-Myers Squibb (BMS), GlaxoSmithKline, Merck (and others) to push deadly HIV drugs – usually at taxpayer expense.
Although labs continue to use PointCare Now and its CD4 Gold reagents, the recent FDA warning letters do not appear on the PointCare website or in any of these marketing materials – nor is there any evidence that PointCare, clinical laboratories or advertisers made any attempt to contact patients who are misdiagnosed by the faulty equipment and protocols, poisoned by HAART medications or criminally convicted for having sex.
RELIABILITY
Dr. Marion Joppe describes “The Research Process” this way:
Because of the failure to conduct population studies that would have helped researchers understand CD4+ variability with age, sex, race, time of day or health status; along with the lack of standardization, the reliability of the HIV test results of the absolute CD4+ T cell value by flow cytometry is, at best, wholly unreliable.
While caution must be exercised in the interpretation of unreliable results, this has not been emphasized by the CDC. White blood cell counts can vary substantially from day to day and may account for shifts of as much as 50 to 150 in normal adults, although the degree of this change may be less in individuals with lower CD4 counts. Also substantial variability exists from laboratory to laboratory; those that do not perform cell count procedures frequently – or do not have quality assurance programs – can be expected to produce inaccurate test results. Compounding this problem, an extended delay of more than 48 hours between the time of sampling and actual specimen processing will result in inaccurate values. Therefore, if a laboratory does not perform the test on a daily basis, or if, for example, blood is drawn on Friday and processed on Monday, the test results may be inaccurate. Another common source of inaccuracy is refrigeration of the blood sample.
Because caution conflicts with their ongoing social marketing campaigns, the CDC ignores the known science of CD4+T cell subsets, the Th1/Th2 dual strategy of the immune system, the function of nitric oxide in cell mediated immunity and the possibility for reversal of this immune imbalance with inexpensive nutracuticals, antioxidants and detoxification.
During the two years of its involvement in HIV-related criminal cases and its review of medical and laboratory records, OMSJ has found no evidence that US laboratory facilities exercise caution in the use of flow cytometry equipment or have met any of the CDC’s recommended standards. Instead, laboratories shroud their operations in secrecy, which is only exposed when laboratories like Quest Diagnostics pay $241 million in fines for fraud and kickbacks or $302 million for illegally marketing misbranded diagnostic equipment.
Given these findings, there is no credible evidence that HIV is responsible for the decrease in CD4+T cells in acquired immune deficiency or that a decrease in CD4+T cells is a unique finding in people who are HIV+: Nor is there any credible evidence that the current usage of flow cytometry technology is justified as a diagnostic therapeutic tool in the current HIV/AIDS paradigm. Unfortunately, it is unlikely that clinicians will abandon these voodoo technologies anytime soon.
Dr. Nancy Banks is a graduate of Hunter College and Harvard Medical School. She practiced general obstetrics and gynecology for 25 years and is an expert in the testing, diagnosis, and treatment of sexually-transmitted diseases. She is the author of the award-winning book AIDS, Opium, Diamonds & Empire (2010). Clark Baker is OMSJ’s Principal Investigator.
.
PRODUCT RECALLS
Since 2004, the FDA has issued 66 recalls of flow cytometry products, devices, components and computer software.
The FDA has issued numerous warning letters to PointCare Technologies, a leading developer and producer of flow cytometry products. In its latest warning letter dated 14 June 2011, the FDA cited PointCare’s repeated and unresolved problems with their testing equipment, reagents and manufacturing facilities:
“…two of these three lots failed the specifications for osmolality and optical density (OD)… devices are adulterated… their manufacture, packing, storage, or installation are not in conformity with the Current Good Manufacturing Practice (CGMP) requirements… did not perform adequate stability studies after changing the packaging of CD4NOW Gold Reagent to determine an accurate shelf-life for the product… functional performance of the gold pack was not acceptable… failed to provide scientific justification for performing gold functional testing on only one vial per lot… Failure to establish, maintain, and implement a corrective and preventative action procedure… due to an AC charging cable/adapter bursting into flames while service personnel charged the unit… Failure to have quality audits conducted by an individual that does not have direct responsibility over the matters being audited…” (emphasis added)THE MARKETING OF JUNK SCIENCE
Of the 66 aforementioned recalls and warning letters, FDA complaints were issued for failures that typically result in low CD4 counts. For agencies that need low counts to claim high HIV infection rates e.g. revenues (and for clinicians who accept kickbacks and bribes from companies like Bristol-Myers Squibb [BMS] and Gilead Sciences), flow cytometry helps clinicians justify the unnecessary delivery of toxic HAART therapies to healthy patients.
Flow cytometry is especially helpful in places like Africa, where mining companies routinely dump toxins and heavy metals into waterways.
One company is Kilembe Mines Limited, which is trying to sell an operation that has polluted the environment and water supplies of southwestern Uganda since 1956. Because foreign investors are reluctant to assume liability that comes with the purchase of toxic waste dumps, clinics and medical devices that blame pollution-caused ailments on HIV offer significant advantages to prospective investors.
According to this report, the Uganda Catholic Medical Bureau (UCMB) has distributed drugs in this area for 30 years.
In an effort to “scale up access to antiretroviral therapy…” UCMB advised that “treatment procedures and the monitoring of clients are simplified so that lower cadres of health workers can be trained to carry out some of the simpler functions… (of HIV testing, diagnosis and treatment).”
Gloria Kakuru of the Baylor University’s International Pediatric AIDS Initiative (BIPAI) explains how PointCare Now helps these “clinicians” diagnose HIV in the area surrounding the Kilembe mines – claiming that the device is superior because it justifies earlier initiation of HAART medication in adults and children:
These inflated misdiagnoses are then used by agencies like UNAIDS, the World Health Organization (WHO) and drug companies like Bristol-Myers Squibb (BMS), GlaxoSmithKline, Merck (and others) to push deadly HIV drugs – usually at taxpayer expense.
Although labs continue to use PointCare Now and its CD4 Gold reagents, the recent FDA warning letters do not appear on the PointCare website or in any of these marketing materials – nor is there any evidence that PointCare, clinical laboratories or advertisers made any attempt to contact patients who are misdiagnosed by the faulty equipment and protocols, poisoned by HAART medications or criminally convicted for having sex.
RELIABILITY
Dr. Marion Joppe describes “The Research Process” this way:
“The extent to which results are consistent over time and an accurate representation of the total population under study is referred to as reliability. In other words, if the results of a study can be reproduced under a similar methodology, then the research instrument is considered to be reliable.”Theoretically, reliable tests should deliver the same results no matter how many times it is applied to random members of the same target groups.
Because of the failure to conduct population studies that would have helped researchers understand CD4+ variability with age, sex, race, time of day or health status; along with the lack of standardization, the reliability of the HIV test results of the absolute CD4+ T cell value by flow cytometry is, at best, wholly unreliable.
While caution must be exercised in the interpretation of unreliable results, this has not been emphasized by the CDC. White blood cell counts can vary substantially from day to day and may account for shifts of as much as 50 to 150 in normal adults, although the degree of this change may be less in individuals with lower CD4 counts. Also substantial variability exists from laboratory to laboratory; those that do not perform cell count procedures frequently – or do not have quality assurance programs – can be expected to produce inaccurate test results. Compounding this problem, an extended delay of more than 48 hours between the time of sampling and actual specimen processing will result in inaccurate values. Therefore, if a laboratory does not perform the test on a daily basis, or if, for example, blood is drawn on Friday and processed on Monday, the test results may be inaccurate. Another common source of inaccuracy is refrigeration of the blood sample.
Because caution conflicts with their ongoing social marketing campaigns, the CDC ignores the known science of CD4+T cell subsets, the Th1/Th2 dual strategy of the immune system, the function of nitric oxide in cell mediated immunity and the possibility for reversal of this immune imbalance with inexpensive nutracuticals, antioxidants and detoxification.
During the two years of its involvement in HIV-related criminal cases and its review of medical and laboratory records, OMSJ has found no evidence that US laboratory facilities exercise caution in the use of flow cytometry equipment or have met any of the CDC’s recommended standards. Instead, laboratories shroud their operations in secrecy, which is only exposed when laboratories like Quest Diagnostics pay $241 million in fines for fraud and kickbacks or $302 million for illegally marketing misbranded diagnostic equipment.
Given these findings, there is no credible evidence that HIV is responsible for the decrease in CD4+T cells in acquired immune deficiency or that a decrease in CD4+T cells is a unique finding in people who are HIV+: Nor is there any credible evidence that the current usage of flow cytometry technology is justified as a diagnostic therapeutic tool in the current HIV/AIDS paradigm. Unfortunately, it is unlikely that clinicians will abandon these voodoo technologies anytime soon.
Dr. Nancy Banks is a graduate of Hunter College and Harvard Medical School. She practiced general obstetrics and gynecology for 25 years and is an expert in the testing, diagnosis, and treatment of sexually-transmitted diseases. She is the author of the award-winning book AIDS, Opium, Diamonds & Empire (2010). Clark Baker is OMSJ’s Principal Investigator.
.
- Mr. Bill
- 02-06-2012, 12:06 AM
How the HIV Papers were fixed at the last moment.
by Janine Roberts
The very papers [were] found to be riddled with fraud...
...I was unused to the idea that I could trust only parts of scientific papers, but this was what I was expected to do. The prestigious investigations and institutions were all in agreement. They condemned as false Gallo's claim that he and his team had isolated this virus in 1982, in other words, before the French. Instead they scathingly concluded that, as of the 22nd February 1984, that is six weeks before these Science papers went for publication on March 30th, Gallo could not have identified HIV, since up until this date ‘no HIV-specific reagents [antibodies] were available to prove that a particular sample harboured the AIDS virus.'
In other words, Gallo could not have identified HIV in 1982 and 1983 as he has claimed, by detecting antibodies specific to it. The investigating scientists pointed out that it was impossible to prove an antibody targeted the AIDS virus before proving what virus caused AIDS!
rest of story: http://fearoftheinvisible.com/how-th...me-to-be-fixed
Senior Investigators - 'HIV scientific papers riddled with Errors'
The CDC, America's foremost disease control institution, currently acknowledges: ‘Four papers from Dr Gallo's laboratory, demonstrating that HTLV-III retrovirus was the cause of AIDS, were published in Science in May 1984'.
I needed to understand these key experiments - and this task would surely be made easier since, not only did I have the Science papers, but the related laboratory documents unearthed by the above scientific and Congressional investigations and by John Crewdson. These included original research notebooks, drafts of key papers, laboratory correspondence, all relating to the discovery of HIV. It was a priceless resource that would surely give me all I needed.
I soon discovered that there had been astonishingly five major investigations between 1990 and 1995 into possible fraud in Gallo's HIV research, several of these overlapping with the others. The first was the one that I have already mentioned, run by the NIH's Office of Scientific Integrity (OSI) and the Richard's Panel. Its goals, set in October 1990, were to focus ‘particularly' on the integrity of the first of the four papers published in Science in May 1984, the one on which Popovic was the lead author, since this paper described the key experiments cited in the application for a Patent on the HIV Test.
The second inquiry was under a powerful Congressional Investigative Sub-Committee headed by Rep. John Dingell. It would prevent key documents from being shredded by the NIH. The third was under the Inspector General of the Department of Health and examined criminal fraud in the ‘HIV Test' patent application. The fourth was under the Office of Research Integrity of the Department of Health and Human Resources and looked for fraud, deception and ‘scientific misconduct' in the Gallo Science papers. And the fifth and last was by the US Secret Service, the body normally charged with safeguarding the security of the US President. It would check the related laboratory documents in the finest forensic lab in Washington. If any were forged, it would find out.
All together, this was by far the most formidable governmental investigation into the honesty of scientific research ever undertaken.
rest of story: http://fearoftheinvisible.com/aidsresearch
Robert Gallo Defends Himself
I was astonished to receive this email from Gallo while I was researching him in 2006. i had not previously been in touch with him.
From: gallo@umbi.umd.edu
Subject: HIV/AIDS
Dear Ms. Roberts,
Since you obviously have a built-in bias about the causative role of HIV in AIDS and about me, I am not surprised that you didn't interview me nor find out what in the end happened to S. Hadley's report. Even Congressman Dingell ‘disavowed it', and he was going after numerous scientists during that period, and clearly not trying to find anyone free of wrong doing. Even though I'm sure you won't read it I suggest you should take a look at the history myself and Montagnier did (The discovery of HIV as the cause of AIDS. New England Journal of Medicine. 349:2283-2285, 2003.).
I'm sure it also doesn't bother you that Montagnier and I have written the history of these events twice, the last being in the New England Journal of Medicine in 2003. We have zero differences of views in the history, not even one comma. I think we are the ones who know the facts and not you and Ms. Hadley who had fed a reporter and some others the nonsense you willingly and I think eagerly swallow. You may not understand that Ms. Hadley is not a scientist, was bent on finding wrong doing, (note there were hundreds of millions of dollars involved in the patent we won for the U.S., and much of that money went to U.S. lawyers representing the French group). I have my thoughts about Ms. Hadley's relationships with these people. Most of us also find Ms. Hadley to be a quite unusual person. Let me leave it at that and the fact that she was ultimately disqualified as a ‘qualified' witness.
Of all your libelous, vicious untruths the most bizarre is that the Secret Service found evidence of ‘forgery' in our books. This is a Hadley fantasy. The Secret Service openly denied any significance of Hadley's putative ‘findings'. Moreover, if we were of that kind of people who would do forgery, do you believe we would be stupid enough to then hand over our books? Be aware that in those days no one was even required to keep such records, and if one did, holding on to the books was usually not longer than three years. Hadley was doing her unprofessional work some 6-7 years after those events.
I'm sure you do not care but people suffered a great deal in that period. Scientists like Nobelist David Baltimore and his associates and collaborators; the great cancer physician - Bernie Fisher; Popovic, myself, and many many more scientists during Hadley's witch hunts and, of course, our families. Needless to say medical research from many groups was stopped for 4-6 years. This is the true scandal - not the issues you consciously or unconsciously have so distorted. Your writing is as vicious and slanderous as anything I have witnessed. One hopes in vain that if you do not understand the issues or history, you would at least have some human decency and correct what you wrote.
Sincerely,
Robert C. Gallo
Director
Institute of Human Virology
University of Maryland Baltimore
725 W. Lombard Street
Suite S307
Baltimore, MD 21201
phone: 410-706-8614
fax: 410-706-1952
email: gallo@umbi.umd.edu
www.ihv.org
[call the Secret Service]
I was not only amazed to receive this - I was astonished by what Gallo had chosen to object to. He did not object to what I wrote about the last minute changes he made to the Popovic paper, nor did he object to my citations from the devastating conclusions of the ORI or from the Inspector General's investigations into his work, but only to my mention of what the Secret Service had discovered in his papers.
After receiving this, I thought it best to check what the Secret Service had to say, to make sure I had not made any errors. I have previously investigated many intelligence operations. This has given me contacts I can call on when needed. Within three days, I had the former Head of the Secret Service on the phone, Larry Stewart, the man who had led their investigation into Robert Gallo's HIV research.
He confirmed that they found convincing evidence that many of Gallo's laboratory documents were ‘fixed' prior to being presented as evidence `- and were thus fraudulent.
But I was not the first to cite these Secret Service findings. They are also presented in some detail in John Crewdson's 2003 book, Science Fictions, as Gallo surely must have known. If he had solid legal objections to my brief mention of the Secret Service findings, then I am sure he would have taken it up earlier with Crewdson.
Crewdson detailed how the Secret Service's forensic laboratory had proved that laboratory records presented as evidence by Gallo were fraudulently created, not on the date stated, not when the experiment was done, but later. This was particularly obvious in one document dated 1984 that reported the use of ‘HIV' - years before the virus was given this name.
As for Gallo saying ‘Moreover, if we were of that kind of people who would do forgery, do you believe we would be stupid enough to then hand over our books?" Gallo had no choice. He had to produce his records in 1985-6. He was legally forced to do so by lawyers acting for the French (thus within the three year period he mentioned); then later these same records were retrieved by the NIH and the Congressional Investigation.
But I was most surprised by his defensiveness. It seems that 23 years after he published his research, he still feels highly vulnerable to challenge.
ref: http://fearoftheinvisible.com/gallodefendsemai
.
- Mr. Bill
- 02-06-2012, 01:37 AM
Ok, I am not sure HIV causes AIDS, but I'm not going to believe it because the government says it's so. Government tends to lie to protect their contributors. However, until I am convinced otherwise, I'm going to practice as if HIV does cause AIDS.It's pretty much a given never to take the government's word for anything. But as for the validity of HIV/AIDS, who the hell can you believe? Besides trusting in ''symbols of authority,' what can we fall back on instead - what else can we rely upon?
To be honest, I don't avoid BB because of AIDS. I avoid it because of genital herpes. In fact, I tend to avoid FS for that reason, unless I have a pretty good relationship with the provider.
So, read and learn! The truth will not be harmed by opposing opinions, in fact, what we know is the truth will either be strengthened by opposing opinions, or exposed as not the truth. Both are good outcomes. Originally Posted by CuteOldGuy
- gut instinct
- life experience
- logic
- common sense
If the original truth (about HIV/AIDS) is a lie (the seed), then all subsequent data/proof (the fruit) will also be a lie. It's common logic; you can't get a peach from a thorn tree.
Science today is controlled by special interests and their influence over publication of data - it's all about perception - not unbiased facts - and this control over publication is especially relevant to the HIV/AIDS image.
Think of how you arrived at your conclusion that HIV = AIDS. You must have read about it somewhere, or watched a TV show - over and over again for years on end - you've been bombarded with the inescapable declaration that HIV = DEATH - and likely, queers fucking monkeys are responsible.
Yet when you reverse engineer all the rhetoric - to ask how does anyone get facts on HIV/AIDS - or better yet, where do physicians get their facts, it all leads back to control over publication in scientific journals - which are regarded as the gold standard by doctors, media reporters and on the bottom rung, the general public.
Here is the post which vividly illustrates this problem...
http://www.eccie.net/showthread.php?...60#post2130060
Throughout this thread, I've posted a ton of evidence - which point to a plethora of lies, corruption, greed and malfeasance - that if digested with an open mind should cause the independent thinking man to suspect something is gravely wrong.
You must ask yourself, what do the following traits tell you?
- gut instinct
- life experience
- logic
- common sense
.
- annie@christophers
- 02-06-2012, 02:53 AM
Just want BBBJ.. annie

- Mr. Bill
- 02-06-2012, 03:03 AM
Just want BBBJ.. annie Originally Posted by annie@christophersActually I just want your signature image to go away - it's very irritating.
.
- dilbert firestorm
- 02-06-2012, 03:45 AM
Ok, I am not sure HIV causes AIDS, but I'm not going to believe it because the government says it's so. Government tends to lie to protect their contributors. However, until I am convinced otherwise, I'm going to practice as if HIV does cause AIDS. Originally Posted by CuteOldGuy
FYI, this is just an observation on my part. the corruption of the FDA and its subsidiary agencies like the CDC and the NIH had its start in the late 60's and early 70's when presidents LBJ & Nixon started appointing business men from the medical industries, some of whom may have a medical degrees instead of scientists or doctors as directors of these agencies. they have a different mindset than the scientists or doctors.
Its quite a shift for the FDA since its creation. It went from protecting consumers (which they now do only as a pretense) to protecting industry.
FDA has been noted for its dubious decisions on a number of drugs they okayed only to have to recall them and later ban them from the market.
One of the best examples of FDA protecting the industry is the way they prevented/disallowed the introduction of Truvia (It is a natural sweetener that is 1000 times sweeter than cane sugar and comes from a plant grown in South America) in the U.S. in 1982. They ignored the fact that this product was safely used in japan for 40 years and by South American tribes that used it for centuries. They basically said that this product was not safe and further studies were needed. Its only the last 3 years that the FDA changed it stance over allowing Truvia, they added a condition that a sugar componet like frutose or sucrose be added to truvia. product was suppressed by request in a sealed complaint to the FDA. Its alleged that the complainant was either the artificial sweetener manufactures or the soft drink companies.
.
its one example that I'm aware of . I'm sure there are better ones.
- Sweet N Little
- 02-06-2012, 08:10 AM
Actually I just want your signature image to go away - it's very irritating.well if that isn't the pot calling the kettle black
. Originally Posted by Mr. Bill

I'll not bother commenting here anymore, I know you have lots of copy and paste work to do


at least put a hot chick on your avatar



- Mr. Bill
- 02-06-2012, 02:30 PM
At the core of diagnosing HIV, the testing methodology itself is flawed - and the conflicts of financial interest are self-evident.
On the very day that Robert Gallo falsely announced he had discovered the cause of AIDS (HIV), both he and the NIH (National Institutes of Health) filed a patent for a HIV test. From that point further the US government itself had a vested interest in the HIV/AIDS paradigm, resulting in hundreds of millions of dollars revenue each year from HIV tests, with Robert Gallo himself receiving substantial royalties each year.
Subsequent investigations into fraud committed by Gallo and the unscrupulous activities by the NIH have been stonewalled, obfuscated and summarily dismissed. HIV/AIDS has thus become a catch-all phrase for fear mongering and profit - that has nothing to do with public health.
Related: http://exlibhollywood.blogspot.com/2...s-jewelry.html
.
On the very day that Robert Gallo falsely announced he had discovered the cause of AIDS (HIV), both he and the NIH (National Institutes of Health) filed a patent for a HIV test. From that point further the US government itself had a vested interest in the HIV/AIDS paradigm, resulting in hundreds of millions of dollars revenue each year from HIV tests, with Robert Gallo himself receiving substantial royalties each year.
Subsequent investigations into fraud committed by Gallo and the unscrupulous activities by the NIH have been stonewalled, obfuscated and summarily dismissed. HIV/AIDS has thus become a catch-all phrase for fear mongering and profit - that has nothing to do with public health.
Related: http://exlibhollywood.blogspot.com/2...s-jewelry.html
.
- Mr. Bill
- 02-06-2012, 02:43 PM
1957 Oscar nominee Rock Hudson was HIV negative

Over fifty years ago, Rock Hudson, born Roy Scherer Jr. in 1925, was nominated for best Performance by an Actor in a Leading Role by the Academy of Motion Picutres, for his portrayal of Jordan 'Bick' Benedict Jr. in the epic film, Giant. George Stevens won the Oscar that year for Best Director.
In the United States, HIV/AIDS became a fashionable Hollywood cause celebre shortly before actor Rock Hudson died in 1985. The unfolding drama was thrust into the bright lights of the public eye when Mr. Hudson succumbed to a series of health complications widely reported as AIDS.
However, a review of available biographical data, and a privately-held, unpublished source, seem to indicate that his death was caused by other factors.
Rock Hudson had quadruple bypass heart surgery made necessary by a diet rich in animal fat, years of alcohol abuse, and a chain-smoking habit that continued even after the bypass surgery. His body's rejection of a blood transfusion given during the bypass, and an experimental AIDS drug called HPA23 given to him by physicians in Paris further eroded his precarious condition.
Despite having put AIDS on the list of Hollywood "cause celebs" and making an indelible mark on the world consciousness of HIV, Mr. Hudson actually tested negative for HIV one year before his death in 1985.

It is ironic that an experimental HIV/AIDS drug was partly responsible for his untimely death when the actor's immune deficiency was not caused by HIV.
Because AIDS is a syndrome, and there has been no irrefutable evidence that HIV causes AIDS, the answer to what causes immune deficiency is revealed in examples such as Rock Hudson's.
One of the irrefutable causes of immune deficiency is actually immune overactivation, which in Mr. Scherer's case, it was his body's rejection of foreign blood he received during a transfusion, added to the already compromised immune system due to alcoholism, a four-pack a day smoking habit, and a quadruple bypass open heart surgery.
Rock Hudson was a major motion picture star during the '60s, and in the '70s he became well-known on the small screen as the star of the television serial, "MacMillan and Wife." During the AIDS hysteria of the '80s, his cameo appearances on Dynasty that featured his character kissing a female costar made headlines.
Rock Hudson's role in Giant was a powerful example of his acting ability, and it was a craft he took very seriously. Perhaps the greatest disappointment towards the end of his life were the mediocre parts he landed both on the silver screen and television. His sexuality and the decline in health overshadowed nearly a half-century of acting.
Now that more than twenty years has passed, there is greater appreciation of his talent, preserved for the ages on film.
ref: http://notaids.com/en/rock
.

Over fifty years ago, Rock Hudson, born Roy Scherer Jr. in 1925, was nominated for best Performance by an Actor in a Leading Role by the Academy of Motion Picutres, for his portrayal of Jordan 'Bick' Benedict Jr. in the epic film, Giant. George Stevens won the Oscar that year for Best Director.
In the United States, HIV/AIDS became a fashionable Hollywood cause celebre shortly before actor Rock Hudson died in 1985. The unfolding drama was thrust into the bright lights of the public eye when Mr. Hudson succumbed to a series of health complications widely reported as AIDS.
However, a review of available biographical data, and a privately-held, unpublished source, seem to indicate that his death was caused by other factors.
Rock Hudson had quadruple bypass heart surgery made necessary by a diet rich in animal fat, years of alcohol abuse, and a chain-smoking habit that continued even after the bypass surgery. His body's rejection of a blood transfusion given during the bypass, and an experimental AIDS drug called HPA23 given to him by physicians in Paris further eroded his precarious condition.
Despite having put AIDS on the list of Hollywood "cause celebs" and making an indelible mark on the world consciousness of HIV, Mr. Hudson actually tested negative for HIV one year before his death in 1985.

It is ironic that an experimental HIV/AIDS drug was partly responsible for his untimely death when the actor's immune deficiency was not caused by HIV.
Because AIDS is a syndrome, and there has been no irrefutable evidence that HIV causes AIDS, the answer to what causes immune deficiency is revealed in examples such as Rock Hudson's.
One of the irrefutable causes of immune deficiency is actually immune overactivation, which in Mr. Scherer's case, it was his body's rejection of foreign blood he received during a transfusion, added to the already compromised immune system due to alcoholism, a four-pack a day smoking habit, and a quadruple bypass open heart surgery.
Rock Hudson was a major motion picture star during the '60s, and in the '70s he became well-known on the small screen as the star of the television serial, "MacMillan and Wife." During the AIDS hysteria of the '80s, his cameo appearances on Dynasty that featured his character kissing a female costar made headlines.
Rock Hudson's role in Giant was a powerful example of his acting ability, and it was a craft he took very seriously. Perhaps the greatest disappointment towards the end of his life were the mediocre parts he landed both on the silver screen and television. His sexuality and the decline in health overshadowed nearly a half-century of acting.
Now that more than twenty years has passed, there is greater appreciation of his talent, preserved for the ages on film.
ref: http://notaids.com/en/rock
.
- Guest070818-1
- 02-06-2012, 04:53 PM
Thanks for posting.