To sum up, there is no definitive evidence or proof that HIV causes AIDS.
In support of the HIV/AIDS theory, we have only Robert Gallo's altered documents, a press conference, continued government collusion, mega media hype and a naive public that thinks its government representatives and the pharmaceutical companies have their best interests in mind.
Once a healthy individual's immune system response is detected, rather than congratulate them, doctors are given the authority to prescribe deadly chemicals that are guaranteed to destroy their immune system and cause death.
After the person succumbs to the poisonous chemical assault, their demise is then blamed on AIDS and everybody cries and wears little ribbons. The real culprit is never suspected nor dealt with.
The HIV virus that causes AIDS is brought to you by the same people who, after billions of tax-payer dollars in funding, didn't bring you the virus that causes cancer. It's the same well-funded circus, just a different act.
...and nothing will ever change until people start thinking for themselves.
.
- Mr. Bill
- 05-03-2012, 10:00 PM
- KCQuestor
- 05-04-2012, 12:26 AM
Wow, you really are a moron, Bill. I was right when I told Questor it was futile, I should have followed my own advice. Originally Posted by Jackie DevlinHAHAHA, I've been biting my tongue all day and you and SillyGirl just look up the banner that I dropped.
Guys, as soon as he cited (I said "cited", not "quoted", you illiterate idiot) Duesberg the game was up. If ever there was a discredited "expert", it is him. And Ben Stein? The guy from "Ferris Bueller" who made a documentary about "intelligent design" is NOT a credible expert on the scientific process.
"Show me the paper. Show me the paper!"
What a ridiculous request. There is no need for a single paper that says "HIV causes AIDS". We have hundreds of thousands of papers that show AIDS is always accompanied by HIV, and when HIV is not present, neither is AIDS. Multiple virological studies show that HIV presence is the ONLY common factor among people with AIDS. Epidemiological studies show that as HIV spreads into a population, so does AIDS. Immunological studies show that when people take drug cocktails that block HIV replication their survival rates increase dramatically, and when their viral loads are lowered they are less likely to develop AIDS. Pediatric studies show that children who have no other environmental or behavioral risk for AIDS are born with HIV from infected mothers, the child develops AIDS.
This is because scientists have areas of specialty, and their research -- and thus the papers they produce -- are extremely specialized. There is no need for a paper that says "HIV causes AIDS"
But you know what, Jerry McGuire? Here is one:
O'Brien, S.J. & Goedert, J.J. (1996). HIV causes AIDS. Current Opinions in Epidemiology, 8(5):613-8.
I didn't want to bring this up, because I know what your response will be. "Durr, that journal has 'Opinions' right in the title! Clearly it is biased." Go ahead. I'll be over here waiting.
Dammit Jackie and SillyGirl. I'm back in it. Fuck me.
- KCQuestor
- 05-04-2012, 12:31 AM
Oh, and feel free to lump me in with all the other scientists that you tarnish because they take money from the government for research grants. Mine comes from the National Science Foundation, not the National Institute of Health, so maybe I'm not as bad as those evil NIH and CDC folks.
- SinsOfTheFlesh
- 05-04-2012, 01:15 AM
Well that's ok Questor. The scientific community has long since moved beyond establishing that HIV causes AIDS. There is now a wealth of research that demonstrates not just that HIV causes AIDS, but explains exactly what HIV does to the body to cause AIDS. We now know that the virus affects more than just the T-cells of the immune system. It also affects bone marrow and red blood cell production, attacks and kills neurons, and affects the hippocampus, the area of the brain involved in memory and spatial relationships, which explains why early onset of Alzheimer's like symptoms are common among AIDS patients, though previously no explanation for why had been available.
If you look a few pages back, I posted half a dozen links to research that goes beyond the proof that HIV causes AIDS, and actually proves HOW HIV causes AIDS. But of course Bill never clicked the links, and even if he did, he wouldn't have understood any of it.
But after begging so earnestly for anyone to provide him with a link, to again post his demand for proof even after I supplied it, relegates Bill to the role of the Black Knight protesting "its merely a flesh wound".
If you look a few pages back, I posted half a dozen links to research that goes beyond the proof that HIV causes AIDS, and actually proves HOW HIV causes AIDS. But of course Bill never clicked the links, and even if he did, he wouldn't have understood any of it.
But after begging so earnestly for anyone to provide him with a link, to again post his demand for proof even after I supplied it, relegates Bill to the role of the Black Knight protesting "its merely a flesh wound".
- Mr. Bill
- 05-04-2012, 02:08 AM
The Alchemy of Flow Cytometry
source: http://www.omsj.org/corruption/the-a...flow-cytometry
Since 2009, OMSJ has examined more than 100 criminal, civil and military cases related to testing, diagnosis and treatment of HIV and AIDS. In the majority of those cases, OMSJ found that clinicians who relied on high patient caseloads to generate revenue routinely use unreliable HIV tests to misdiagnose their patients.

OMSJ has reported the widespread use of kickbacks used to bribe clinicians who unnecessarily prescribe deadly drugs to their healthy patients and found scarce evidence that those clinicians attempted to follow HHS or CDC guidelines to diagnose HIV. By the end of 2011, those kickbacks amounted to more than $750 million, which doesn’t include the millions paid to celebrities like Magic Johnson and Bono, whose propaganda campaign is now the focus of a multi-billion dollar international corruption scandal.
Weeks after testifying against her own patient, Bristol-Myers Squibb (BMS) sent Donna Sweet MD a $2.9 million grant. BMS is the maker of Sustiva (Atripla), which Dr. Sweet unnecessarily prescribed to her asymptomatic patient in 2010.
To justify the illegal marketing and distribution of toxins like AZT, Sustiva and Atripla, clinicians use tests that the FDA has never approved for diagnosing HIV in healthy patients.
DEFINING AIDS
The onset of the “AIDS pandemic” began with a tiny subculture of promiscuous homosexuals, many of whom attended circuit parties where they ingested meth, huffed carcinogens (amyl nitrate) and engaged in hours of unprotected anal sex (called barebacking). The drug-fueled colorectal trauma, injuries and comingling of fecal matter, semen and blood resulted in chronic infections and visits to doctors, who prescribed heavy doses of antibiotics that further compromised their patients’ immune function.
When some well-intended conservatives clumsily declared that the infections were related to “God’s intolerance of homosexuals,” it became politically suicidal to suggest that the self-destructive behavior of barebacking meth addicts had anything to do with the injuries incurred.

While gay activists pressured gullible celebrities into pushing the AIDS propaganda, scientists exploited the politics to secure funding. But despite the hysteria, Americans who didn’t abuse drugs or engage in anal intercourse didn’t acquire AIDS. Among the tiny number of heterosexuals who did, patients like Elizabeth Glazer and Ryan White succumbed only after taking fatal doses of the carcinogenic mutagen, Zidovudine (AZT). Their homicides were then used to promote the myth of heterosexual AIDS.
Despite the hysteria, actual AIDS cases were so rare that the CDC invented the Coolfont Estimate, which estimated the number of HIV infected persons in the U.S. They arrived at an estimate of between 1-1.5 million infections – a number extrapolated from the Kinsey Report, which estimated the number of homosexuals, IV drug users, hemophiliacs and heterosexuals who allegedly took part in homosexual acts in 1948.
Although the US population swelled from 228 million to more than 312 million since the alleged pandemic began, the CDC’s latest reports show that the initial estimates have remain unchanged at 1.2 million; a number that – if true – represents one-third of one percent of the total US population that (coincidentally) engages in self-destructive behavior.
To inflate these unremarkable numbers, the CDC changed the AIDS surveillance case definition “to include all HIV-infected persons who have less than 200 CD4+ T-lymphocytes/uL.” This change meant that – regardless of the incompetence of HIV tests – a lab that reports CD4 counts below 200 will, by definition, condemn healthy patients to a diagnosis of “full-blown AIDS” and a lifetime of deadly drugs that poisons patients like these: click here to see accompanying video
.
source: http://www.omsj.org/corruption/the-a...flow-cytometry
Since 2009, OMSJ has examined more than 100 criminal, civil and military cases related to testing, diagnosis and treatment of HIV and AIDS. In the majority of those cases, OMSJ found that clinicians who relied on high patient caseloads to generate revenue routinely use unreliable HIV tests to misdiagnose their patients.
by NANCY BANKS, MD & CLARK BAKER

OMSJ has reported the widespread use of kickbacks used to bribe clinicians who unnecessarily prescribe deadly drugs to their healthy patients and found scarce evidence that those clinicians attempted to follow HHS or CDC guidelines to diagnose HIV. By the end of 2011, those kickbacks amounted to more than $750 million, which doesn’t include the millions paid to celebrities like Magic Johnson and Bono, whose propaganda campaign is now the focus of a multi-billion dollar international corruption scandal.
Weeks after testifying against her own patient, Bristol-Myers Squibb (BMS) sent Donna Sweet MD a $2.9 million grant. BMS is the maker of Sustiva (Atripla), which Dr. Sweet unnecessarily prescribed to her asymptomatic patient in 2010.
To justify the illegal marketing and distribution of toxins like AZT, Sustiva and Atripla, clinicians use tests that the FDA has never approved for diagnosing HIV in healthy patients.
DEFINING AIDS
The onset of the “AIDS pandemic” began with a tiny subculture of promiscuous homosexuals, many of whom attended circuit parties where they ingested meth, huffed carcinogens (amyl nitrate) and engaged in hours of unprotected anal sex (called barebacking). The drug-fueled colorectal trauma, injuries and comingling of fecal matter, semen and blood resulted in chronic infections and visits to doctors, who prescribed heavy doses of antibiotics that further compromised their patients’ immune function.
When some well-intended conservatives clumsily declared that the infections were related to “God’s intolerance of homosexuals,” it became politically suicidal to suggest that the self-destructive behavior of barebacking meth addicts had anything to do with the injuries incurred.

While gay activists pressured gullible celebrities into pushing the AIDS propaganda, scientists exploited the politics to secure funding. But despite the hysteria, Americans who didn’t abuse drugs or engage in anal intercourse didn’t acquire AIDS. Among the tiny number of heterosexuals who did, patients like Elizabeth Glazer and Ryan White succumbed only after taking fatal doses of the carcinogenic mutagen, Zidovudine (AZT). Their homicides were then used to promote the myth of heterosexual AIDS.
Despite the hysteria, actual AIDS cases were so rare that the CDC invented the Coolfont Estimate, which estimated the number of HIV infected persons in the U.S. They arrived at an estimate of between 1-1.5 million infections – a number extrapolated from the Kinsey Report, which estimated the number of homosexuals, IV drug users, hemophiliacs and heterosexuals who allegedly took part in homosexual acts in 1948.
Although the US population swelled from 228 million to more than 312 million since the alleged pandemic began, the CDC’s latest reports show that the initial estimates have remain unchanged at 1.2 million; a number that – if true – represents one-third of one percent of the total US population that (coincidentally) engages in self-destructive behavior.
To inflate these unremarkable numbers, the CDC changed the AIDS surveillance case definition “to include all HIV-infected persons who have less than 200 CD4+ T-lymphocytes/uL.” This change meant that – regardless of the incompetence of HIV tests – a lab that reports CD4 counts below 200 will, by definition, condemn healthy patients to a diagnosis of “full-blown AIDS” and a lifetime of deadly drugs that poisons patients like these: click here to see accompanying video
.
- Mr. Bill
- 05-04-2012, 02:11 AM
The Alchemy of Flow Cytometry
source: http://www.omsj.org/corruption/the-a...flow-cytometry
In one of OMSJ’s recent cases, a criminal defendant was charged with numerous counts of having exposed others to HIV. Although the HIV tests could be easily challenged, the fact that his lab reports showed CD4 counts as low as 32 and 7 suggested that he only had a few months to live – a fact refuted by his undeniable health.
For this reason, OMSJ began an investigation into the reliability and use of flow cytometry as it applies to HIV and AIDS.
FLOW CYTOMETRY
A careful reading of the medical and scientific journals establishes little correlation between CD4 cells and HIV. In one report, NIAID Director Anthony Fauci explained:
Several years later, Fauci added:
The aggregate CD4+T cell count is measured by a process known as flow cytometry – a measurement of characteristics of single cells suspended in a flowing saline stream moving through a beam of light. Many scientific procedures involve obtaining measurements as average values for the whole population, which differs from flow cytometric analysis measurements that are made on individual particles within the suspension.
Additionally, several parameters can be measured on tens of thousands of individual cells within a few minutes: relative size, relative granularity or internal complexity, and relative fluorescence intensity. Any suspended particle or cell from 0.2-150 micrometers in size is suitable for analysis. The noted characteristics are determined using an optical-to-electronic coupling system that records how the cell or particle scatters incident laser light and emits fluorescence.
However, flow cytometry has numerous intrinsically fatal shortcomings:
1. The fluidics system transports particles in a stream to the laser beam for interrogation.
2. The optics system consists of lasers to illuminate the particles in the sample stream and optical filters to direct the resulting light signals to the appropriate detectors.
3. The electronics system converts the detected light signals into electronic signals that can be processed by a computer. For some instruments equipped with a sorting feature, the electronics system is also capable of initiating sorting decisions to charge and deflect particles.
Flow cytometry evolved from the development of several fields: microscopy; dye chemistry; electronics; and computers. It was the fusion and advances of these diverse technologies that allowed for the evolution of flow cytometry.
.
source: http://www.omsj.org/corruption/the-a...flow-cytometry
In one of OMSJ’s recent cases, a criminal defendant was charged with numerous counts of having exposed others to HIV. Although the HIV tests could be easily challenged, the fact that his lab reports showed CD4 counts as low as 32 and 7 suggested that he only had a few months to live – a fact refuted by his undeniable health.
For this reason, OMSJ began an investigation into the reliability and use of flow cytometry as it applies to HIV and AIDS.
FLOW CYTOMETRY
A careful reading of the medical and scientific journals establishes little correlation between CD4 cells and HIV. In one report, NIAID Director Anthony Fauci explained:
“Although most studies necessarily focus on HIV infection of peripheral-blood mononuclear cells, the lymphocytes that are in the peripheral blood at any given time represent only about 2 percent of the total lymphocyte pool, most of which is in the lymphoid organs. Hence, in certain pathologic processes involving lymphoic cells, the peripheral blood may not accurately reflect the status of disease. Specific immune responses are generated predominantly in lymphoid organs rather than in the peripheral blood.”By analogy, the absence of policemen at a city park does not necessarily mean that the park is unsafe. The park may be safer than a disturbance where policemen are found in larger numbers. While the overall numbers of policemen won’t change in a city, their deployment depends upon when and where they are needed.
Several years later, Fauci added:
“…the primary mechanisms of CD4+T cell depletion in vivo remain unclear; there is no direct evidence that HIV is cytopathic in vivo, despite the fact that cytopathicity can be readily demonstrated in the artificial milieu of culture.”Some researchers have identified numerous subsets of CD4 cells, while others have found that low T-cell numbers are routinely found among life insurance applicants and African populations. Others have found viral load and flow cytometry equally dubious.
The aggregate CD4+T cell count is measured by a process known as flow cytometry – a measurement of characteristics of single cells suspended in a flowing saline stream moving through a beam of light. Many scientific procedures involve obtaining measurements as average values for the whole population, which differs from flow cytometric analysis measurements that are made on individual particles within the suspension.
Additionally, several parameters can be measured on tens of thousands of individual cells within a few minutes: relative size, relative granularity or internal complexity, and relative fluorescence intensity. Any suspended particle or cell from 0.2-150 micrometers in size is suitable for analysis. The noted characteristics are determined using an optical-to-electronic coupling system that records how the cell or particle scatters incident laser light and emits fluorescence.
However, flow cytometry has numerous intrinsically fatal shortcomings:
- While the observed cell stream is three dimensional, the scatter pattern that is generated on computer print-out of the cellular density stream is measured in two dimensions.
- The ability to identify live individual cells of a particular type from dead cells, clumps of cells and debris by a process known as gating is limited by the training and expertise of the observer-technician.
- There are no established standards for the technology of operators. Procedures vary between each technician and lab.
- All FDA-approved flow cytometry devices are based upon “predicate devices” technologies that were marketed before May 28, 1976.
- Researchers claim that the distinguishing characteristics of live individual cells from dead cells and debris can be accurately preserved following paraformaldehyde fixation (which kills all of the cells).
1. The fluidics system transports particles in a stream to the laser beam for interrogation.
2. The optics system consists of lasers to illuminate the particles in the sample stream and optical filters to direct the resulting light signals to the appropriate detectors.
3. The electronics system converts the detected light signals into electronic signals that can be processed by a computer. For some instruments equipped with a sorting feature, the electronics system is also capable of initiating sorting decisions to charge and deflect particles.
Flow cytometry evolved from the development of several fields: microscopy; dye chemistry; electronics; and computers. It was the fusion and advances of these diverse technologies that allowed for the evolution of flow cytometry.
.
- Mr. Bill
- 05-04-2012, 02:14 AM
The Alchemy of Flow Cytometry
source: http://www.omsj.org/corruption/the-a...flow-cytometry
Modern flow cytometry started at the Los Alamos National Laboratories in New Mexico and entered the marketplace by the mid-1970s. After scientists alleged in 1984 that HIV was killing CD4+T cells, researchers developed an advancement they called immunophenotyping.
Immunophenotyping is the analysis of heterogeneous populations of cells for the purpose of identifying the presence and proportions of the various populations of interest. Antibodies are used to identify cells by detecting specific antigens expressed by these cells, which are known as markers. These markers are usually functional membrane proteins involved in cell communication, adhesion, or metabolism. Immunophenotyping using flow cytometry has become the method of choice in identifying and sorting cells within complex populations and is used extensively in the diagnosis and management of AIDS. However, as noted above, the CD4+T cell population is not uniform and the nature of the Th1/Th2 shift has more to do with the development of AID than the aggregate decline of these cells.
FROM RESEARCH TO JUNK SCIENCE
In 1972, Congress established the Office of Technology Assessment (OTA) to serve the legislative branch as an independent source of information and analysis about complex scientific and technical issues. OTA construed health technology broadly, including “all elements of medical practice that are knowledge-based, including hardware (equipment and facilities) and software (knowledge skills)… the set of techniques, drugs, equipment, and procedures used by health care professionals in delivering medical care to individuals and the systems within which such care is delivered.”
By 1978, the OTA produced a shattering report on the state of scientific medicine, Assessing the Efficacy and Safety of Medical Technologies. The report stated:
The U.S. News and World Report issue of 23 November 1987 raised further questions about HIV tests:
In 1996, Congress disbanded the OTA, leading to the systematic deregulation of the various medical technology industries. The U.S. Supreme Court sealed the fate of future scientific transparency by ruling in favor or corporate interests in the Citizens United v. Federal Election Commission (2010).
The OTA’s demise closed a low-budget item that gave Americans too much information to make informed choices. It paved the way for the establishment of a medical and scientific knowledge monopoly that is now permeated by corruption and fraud (junk science).
As a result, the “AIDS industry” continues to use unproven technologies like flow cytometry to diagnose, alarm, and treat healthy people with toxic and expensive drugs that are designed to make them sick (see video).
Fundamental issues regarding the limitations of flow cytometry technology and the propriety of its use in the diagnosis of acquired immune deficiency have never been addressed:
Police officers also use RADAR to enforce basic speed laws. But like GC, officers do not rely upon RADAR devices to enforce laws. Instead, officers rely upon their training and expertise to estimate intoxication and velocities. Once they develop articulable facts that indicate impairment or an unsafe speed, they use GC and RADAR to confirm what their training, expertise and observations initially tell them.
Unlike GC and RADAR, flow cytometry manufacturers compensate for their inaccuracies by inventing proprietary algorithms to report spurious and unreliable cell counts. There is no reliable method for counting standardization for products and operators, and the substantial deviations in technical competency and quality control of test samples between labs render these tests wholly unreliable. Clinicians simply order blood draws and presume that lab results are accurate and meaningful. Clinicians who receive kickbacks from labs and drug companies have little incentive to question results of their asymptomatic patients.
This protocol is akin to policemen who stop 35 mph motorists because their RADAR device captured a 90mph reading. But while police agencies would train or terminate such derelict employees, this practice – when applied to biological testing and flow cytometry – is considered the “medical standard of care.”
.
source: http://www.omsj.org/corruption/the-a...flow-cytometry
Modern flow cytometry started at the Los Alamos National Laboratories in New Mexico and entered the marketplace by the mid-1970s. After scientists alleged in 1984 that HIV was killing CD4+T cells, researchers developed an advancement they called immunophenotyping.
Immunophenotyping is the analysis of heterogeneous populations of cells for the purpose of identifying the presence and proportions of the various populations of interest. Antibodies are used to identify cells by detecting specific antigens expressed by these cells, which are known as markers. These markers are usually functional membrane proteins involved in cell communication, adhesion, or metabolism. Immunophenotyping using flow cytometry has become the method of choice in identifying and sorting cells within complex populations and is used extensively in the diagnosis and management of AIDS. However, as noted above, the CD4+T cell population is not uniform and the nature of the Th1/Th2 shift has more to do with the development of AID than the aggregate decline of these cells.
FROM RESEARCH TO JUNK SCIENCE
In 1972, Congress established the Office of Technology Assessment (OTA) to serve the legislative branch as an independent source of information and analysis about complex scientific and technical issues. OTA construed health technology broadly, including “all elements of medical practice that are knowledge-based, including hardware (equipment and facilities) and software (knowledge skills)… the set of techniques, drugs, equipment, and procedures used by health care professionals in delivering medical care to individuals and the systems within which such care is delivered.”
By 1978, the OTA produced a shattering report on the state of scientific medicine, Assessing the Efficacy and Safety of Medical Technologies. The report stated:
“Evidence indicates that many technologies are not adequately assessed before they enjoy widespread use… Many technologies which have been used extensively have later been shown to be of limited usefulness”…and ” … only 10 to 20 percent of all procedures currently used in medical practice have been shown to be efficacious by controlled trial.”The report implied that 80% to 90% of all routinely-performed procedures are unproven – a conclusion that implicates the technology of flow cytometry that uses immunophenotyping to identify antigen markers on various cell populations.
The U.S. News and World Report issue of 23 November 1987 raised further questions about HIV tests:
“With public health officials and politicians thrashing out who should be tested for HIV, the accuracy of the test itself has been ignored. A study last month by the Congressional Office of Technology Assessment found that HIV tests can be very inaccurate indeed. For groups at very low risk – people who do not use IV drugs or have sex with gay or bisexual men – 9 in 10 positive findings are called false positives, indicating infection where none exists.”OTA’s warning continues to be ignored by the medical and scientific communities and the politicians, agencies and regulators that enable them.
In 1996, Congress disbanded the OTA, leading to the systematic deregulation of the various medical technology industries. The U.S. Supreme Court sealed the fate of future scientific transparency by ruling in favor or corporate interests in the Citizens United v. Federal Election Commission (2010).
The OTA’s demise closed a low-budget item that gave Americans too much information to make informed choices. It paved the way for the establishment of a medical and scientific knowledge monopoly that is now permeated by corruption and fraud (junk science).
As a result, the “AIDS industry” continues to use unproven technologies like flow cytometry to diagnose, alarm, and treat healthy people with toxic and expensive drugs that are designed to make them sick (see video).
Fundamental issues regarding the limitations of flow cytometry technology and the propriety of its use in the diagnosis of acquired immune deficiency have never been addressed:
- Proof that an exogenous virus uniquely attacks CD4+T cells and is cytopathic (see above discussion). Further, the relationship of the Th1/Th2 balance shift in the CD4+T cell population has been ignored.
- Reproducibility – samples drawn concurrently from one subject should deliver the same results in every machine and laboratory that receives those specimens.
Police officers also use RADAR to enforce basic speed laws. But like GC, officers do not rely upon RADAR devices to enforce laws. Instead, officers rely upon their training and expertise to estimate intoxication and velocities. Once they develop articulable facts that indicate impairment or an unsafe speed, they use GC and RADAR to confirm what their training, expertise and observations initially tell them.
Unlike GC and RADAR, flow cytometry manufacturers compensate for their inaccuracies by inventing proprietary algorithms to report spurious and unreliable cell counts. There is no reliable method for counting standardization for products and operators, and the substantial deviations in technical competency and quality control of test samples between labs render these tests wholly unreliable. Clinicians simply order blood draws and presume that lab results are accurate and meaningful. Clinicians who receive kickbacks from labs and drug companies have little incentive to question results of their asymptomatic patients.
This protocol is akin to policemen who stop 35 mph motorists because their RADAR device captured a 90mph reading. But while police agencies would train or terminate such derelict employees, this practice – when applied to biological testing and flow cytometry – is considered the “medical standard of care.”
.
- Mr. Bill
- 05-04-2012, 02:17 AM
The Alchemy of Flow Cytometry
source: http://www.omsj.org/corruption/the-a...flow-cytometry
Flow cytometry employs two techniques to count cells:
To assure the accuracy and reliability of CD4+T cell test results obtained within individual laboratories and to attempt to assure comparability of results between laboratories, the CDC established a list of standard methods for performing the test, as well as guidelines for quality control and quality assurance. The CDC’s recommendations for flow cytometry apply to laboratory safety, specimen collection, specimen transport, maintenance of specimen integrity, specimen processing, flow cytometer quality control, sample analyses, data analysis, data storage, data reporting and quality assurance.
As can be seen, there are multiple steps in this process, any of which that if violated can lead to a substantial alteration in the test results:
1. Blood collection: The type of vial, the time of day and the temperature at which the specimen is handled can all have an effect on test results. CD4+T cell counts are known to be higher in the afternoon than in the morning – a result of the response to the diurnal variation in steroid production from the adrenal gland.
2. Specimen transport: Was the specimen maintained at room temperature? If the specimen is too hot or too cold, cellular destruction might occur. This can be a major problem for transporting of the specimen outside of the collection facility.
3. Specimen Integrity: When the specimen was received, was it too hot or too cold? Was the blood hemolyzed or frozen? Are there visible clots? Has the specimen been received > 72 hours after collection? If so, the specimen must be rejected.
4. Specimen processing: The test should be run within 48 hours, but no later than 72 hours after drawing the blood. Procedures that must be followed:
5. Gently rocking blood for 5 minutes to ensure uniform sample
6. Pipetting accurate blood volumes; vortex sample tubes to mix the blood and reagents and break up cell aggregates
7. Quality and type of reagent used
8. Incubating tubes in dark during staining procedure
9. A lyse/no wash method which requires following manufacture directions (each manufactures has a different set of directions and a different counting algorithm)
10. An immediate capping and storing all stained samples in the dark under refrigeration until flow cytometric analysis is performed.
11. It is advised that the specimens be stored no more than 24 hours unless the laboratory can demonstrate that scatter and fluorescence patterns do not change for specimens stored for longer periods.
12. Machine calibration: Variations in absolute lymphocyte counts obtained by different automated cell counters exposes another problem. A review of four widely used automated counters indicate that analytic variability in the absolute lymphocyte counts due primarily to method variability, is significant and larger than the variability typically observed on inter-laboratory trials of relative CD4+T cell counts. These method biases cannot easily be reduced by calibration, since the cell classification algorithms are built in features of the various counters.
As can be seen, the process of flow cytometry using immunophenotyping requires not only a sophisticated level of technical skill, but a chain of delivery and processing events that is probably difficult to replicate from lab to lab, but also to substantiate. One study found errors of 18% and 35% of absolute CD4+T cell count, while another study found the inter-laboratory variability so significant that it led to conflicting treatment recommendations.
.
source: http://www.omsj.org/corruption/the-a...flow-cytometry
Flow cytometry employs two techniques to count cells:
- Dual Platform Systems – One component determines cell concentrations, while the second determines the relative number of CD4 and CD8 cells. Unless these two components count a common parameter, dual platform systems cannot accurately correlate the results.
- Single Platform Systems – These platforms are especially designed to count the absolute numbers of antibody-labeled cells. These devices are equipped with multiple sample loader, programming facility and computer support, which removes the need for using two different machine to determine the concentration of CD4 and CD8 cells.
- Standardization – HHS, CDC, NIH and FDA have failed to produce meaningful guidelines for quality control, quality assurance or quality of test reagents.
To assure the accuracy and reliability of CD4+T cell test results obtained within individual laboratories and to attempt to assure comparability of results between laboratories, the CDC established a list of standard methods for performing the test, as well as guidelines for quality control and quality assurance. The CDC’s recommendations for flow cytometry apply to laboratory safety, specimen collection, specimen transport, maintenance of specimen integrity, specimen processing, flow cytometer quality control, sample analyses, data analysis, data storage, data reporting and quality assurance.
As can be seen, there are multiple steps in this process, any of which that if violated can lead to a substantial alteration in the test results:
1. Blood collection: The type of vial, the time of day and the temperature at which the specimen is handled can all have an effect on test results. CD4+T cell counts are known to be higher in the afternoon than in the morning – a result of the response to the diurnal variation in steroid production from the adrenal gland.
2. Specimen transport: Was the specimen maintained at room temperature? If the specimen is too hot or too cold, cellular destruction might occur. This can be a major problem for transporting of the specimen outside of the collection facility.
3. Specimen Integrity: When the specimen was received, was it too hot or too cold? Was the blood hemolyzed or frozen? Are there visible clots? Has the specimen been received > 72 hours after collection? If so, the specimen must be rejected.
4. Specimen processing: The test should be run within 48 hours, but no later than 72 hours after drawing the blood. Procedures that must be followed:
5. Gently rocking blood for 5 minutes to ensure uniform sample
6. Pipetting accurate blood volumes; vortex sample tubes to mix the blood and reagents and break up cell aggregates
7. Quality and type of reagent used
8. Incubating tubes in dark during staining procedure
9. A lyse/no wash method which requires following manufacture directions (each manufactures has a different set of directions and a different counting algorithm)
10. An immediate capping and storing all stained samples in the dark under refrigeration until flow cytometric analysis is performed.
11. It is advised that the specimens be stored no more than 24 hours unless the laboratory can demonstrate that scatter and fluorescence patterns do not change for specimens stored for longer periods.
12. Machine calibration: Variations in absolute lymphocyte counts obtained by different automated cell counters exposes another problem. A review of four widely used automated counters indicate that analytic variability in the absolute lymphocyte counts due primarily to method variability, is significant and larger than the variability typically observed on inter-laboratory trials of relative CD4+T cell counts. These method biases cannot easily be reduced by calibration, since the cell classification algorithms are built in features of the various counters.
As can be seen, the process of flow cytometry using immunophenotyping requires not only a sophisticated level of technical skill, but a chain of delivery and processing events that is probably difficult to replicate from lab to lab, but also to substantiate. One study found errors of 18% and 35% of absolute CD4+T cell count, while another study found the inter-laboratory variability so significant that it led to conflicting treatment recommendations.
.
- Mr. Bill
- 05-04-2012, 02:21 AM
The Alchemy of Flow Cytometry
source: http://www.omsj.org/corruption/the-a...flow-cytometry
PRODUCT RECALLS
Since 2004, the FDA has issued 66 recalls of flow cytometry products, devices, components and computer software.
Examples:
COMPANY: BD Biosciences
PRODUCT: FACSDiva Software
REASON: When data file containing one or no fluorescence (SIC) parameters is exported, the software will automatically apply compensation to this file and all subsequently exported files.
UNITS: 1074, US and worldwide distribution
-----------
COMPANY: Quantimetrix Synovialscopics
PRODUCT: Synovial Fluid Control
REASON: This recall was initiated due to efficacy concerns with the stabilized erythrocytes, leukocytes and lymphocytes contained in this product group
UNITS: 24,937 Nationwide distribution
-----------
COMPANY: Cytosol Opthalmics
PRODUCT: ShellGell Sodium Hyaluronate 0.8mL syringe, 12 mg/ml.
REASON: Product sterility may be compromised due to incomplete heat seals in the cannula pouches that are included with the viscoelastic syringe.
UNITS: 3,720 California and North Carolina
-----------
COMPANY: Beckman Coulter, Inc.
PRODUCT: Cyto-Stat/Coulter Cone B6-RD
REASON: Diminished expression on B-cell population when drawn in EDTA tubes, which may lead to inaccurate interpretation of phenotype results
UNITS: 1053 Nationwide and Canada
The FDA has issued numerous warning letters to PointCare Technologies, a leading developer and producer of flow cytometry products. In its latest warning letter dated 14 June 2011, the FDA cited PointCare’s repeated and unresolved problems with their testing equipment, reagents and manufacturing facilities:
Of the 66 aforementioned recalls and warning letters, FDA complaints were issued for failures that typically result in low CD4 counts. For agencies that need low counts to claim high HIV infection rates e.g. revenues (and for clinicians who accept kickbacks and bribes from companies like Bristol-Myers Squibb [BMS] and Gilead Sciences), flow cytometry helps clinicians justify the unnecessary delivery of toxic HAART therapies to healthy patients.
Flow cytometry is especially helpful in places like Africa, where mining companies routinely dump toxins and heavy metals into waterways.
One company is Kilembe Mines Limited, which is trying to sell an operation that has polluted the environment and water supplies of southwestern Uganda since 1956. Because foreign investors are reluctant to assume liability that comes with the purchase of toxic waste dumps, clinics and medical devices that blame pollution-caused ailments on HIV offer significant advantages to prospective investors.
According to this report, the Uganda Catholic Medical Bureau (UCMB) has distributed drugs in this area for 30 years.
In an effort to “scale up access to antiretroviral therapy…” UCMB advised that “treatment procedures and the monitoring of clients are simplified so that lower cadres of health workers can be trained to carry out some of the simpler functions… (of HIV testing, diagnosis and treatment).”
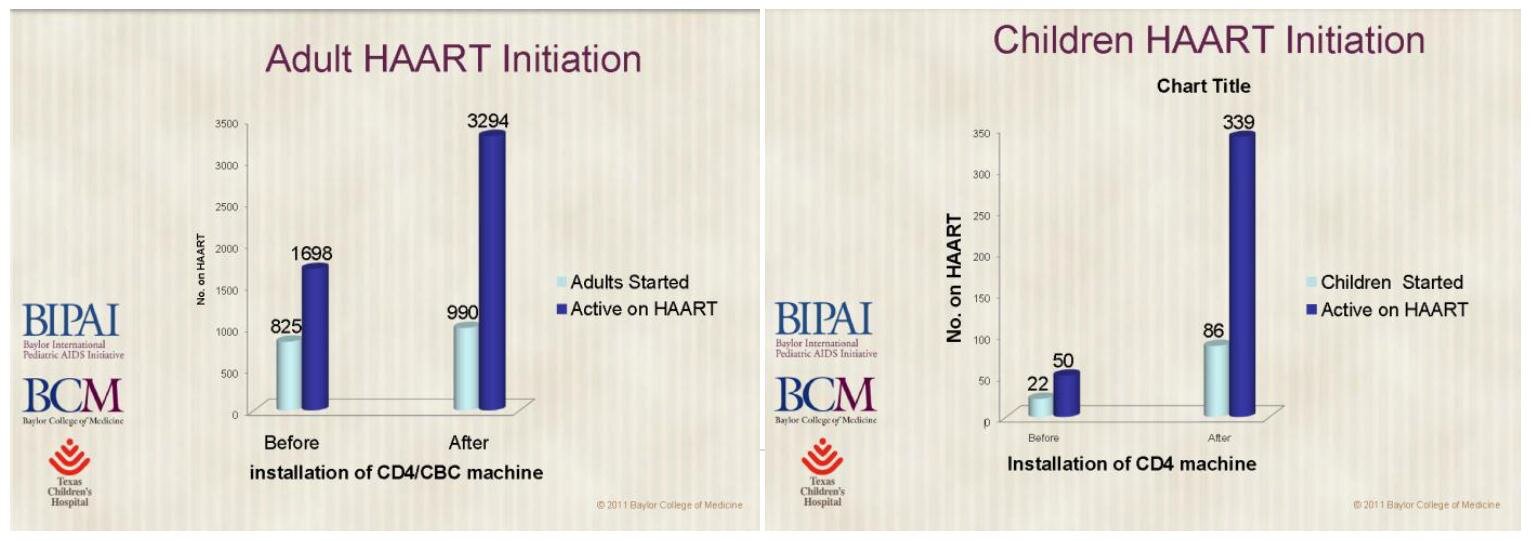
Gloria Kakuru of the Baylor University’s International Pediatric AIDS Initiative (BIPAI) explains how PointCare Now helps these “clinicians” diagnose HIV in the area surrounding the Kilembe mines – claiming that the device is superior because it justifies earlier initiation of HAART medication in adults and children:
These inflated misdiagnoses are then used by agencies like UNAIDS, the World Health Organization (WHO) and drug companies like Bristol-Myers Squibb (BMS), GlaxoSmithKline, Merck (and others) to push deadly HIV drugs – usually at taxpayer expense.
Although labs continue to use PointCare Now and its CD4 Gold reagents, the recent FDA warning letters do not appear on the PointCare website or in any of these marketing materials – nor is there any evidence that PointCare, clinical laboratories or advertisers made any attempt to contact patients who are misdiagnosed by the faulty equipment and protocols, poisoned by HAART medications or criminally convicted for having sex.
RELIABILITY
Dr. Marion Joppe describes “The Research Process” this way:
Because of the failure to conduct population studies that would have helped researchers understand CD4+ variability with age, sex, race, time of day or health status; along with the lack of standardization, the reliability of the HIV test results of the absolute CD4+ T cell value by flow cytometry is, at best, wholly unreliable.
While caution must be exercised in the interpretation of unreliable results, this has not been emphasized by the CDC. White blood cell counts can vary substantially from day to day and may account for shifts of as much as 50 to 150 in normal adults, although the degree of this change may be less in individuals with lower CD4 counts. Also substantial variability exists from laboratory to laboratory; those that do not perform cell count procedures frequently – or do not have quality assurance programs – can be expected to produce inaccurate test results. Compounding this problem, an extended delay of more than 48 hours between the time of sampling and actual specimen processing will result in inaccurate values. Therefore, if a laboratory does not perform the test on a daily basis, or if, for example, blood is drawn on Friday and processed on Monday, the test results may be inaccurate. Another common source of inaccuracy is refrigeration of the blood sample.
Because caution conflicts with their ongoing social marketing campaigns, the CDC ignores the known science of CD4+T cell subsets, the Th1/Th2 dual strategy of the immune system, the function of nitric oxide in cell mediated immunity and the possibility for reversal of this immune imbalance with inexpensive nutracuticals, antioxidants and detoxification.
During the two years of its involvement in HIV-related criminal cases and its review of medical and laboratory records, OMSJ has found no evidence that US laboratory facilities exercise caution in the use of flow cytometry equipment or have met any of the CDC’s recommended standards. Instead, laboratories shroud their operations in secrecy, which is only exposed when laboratories like Quest Diagnostics pay $241 million in fines for fraud and kickbacks or $302 million for illegally marketing misbranded diagnostic equipment.
Given these findings, there is no credible evidence that HIV is responsible for the decrease in CD4+T cells in acquired immune deficiency or that a decrease in CD4+T cells is a unique finding in people who are HIV+: Nor is there any credible evidence that the current usage of flow cytometry technology is justified as a diagnostic therapeutic tool in the current HIV/AIDS paradigm. Unfortunately, it is unlikely that clinicians will abandon these voodoo technologies anytime soon.
Dr. Nancy Banks is a graduate of Hunter College and Harvard Medical School. She practiced general obstetrics and gynecology for 25 years and is an expert in the testing, diagnosis, and treatment of sexually-transmitted diseases. She is the author of the award-winning book AIDS, Opium, Diamonds & Empire (2010). Clark Baker is OMSJ’s Principal Investigator.
.
source: http://www.omsj.org/corruption/the-a...flow-cytometry
PRODUCT RECALLS
Since 2004, the FDA has issued 66 recalls of flow cytometry products, devices, components and computer software.
Examples:
COMPANY: BD Biosciences
PRODUCT: FACSDiva Software
REASON: When data file containing one or no fluorescence (SIC) parameters is exported, the software will automatically apply compensation to this file and all subsequently exported files.
UNITS: 1074, US and worldwide distribution
-----------
COMPANY: Quantimetrix Synovialscopics
PRODUCT: Synovial Fluid Control
REASON: This recall was initiated due to efficacy concerns with the stabilized erythrocytes, leukocytes and lymphocytes contained in this product group
UNITS: 24,937 Nationwide distribution
-----------
COMPANY: Cytosol Opthalmics
PRODUCT: ShellGell Sodium Hyaluronate 0.8mL syringe, 12 mg/ml.
REASON: Product sterility may be compromised due to incomplete heat seals in the cannula pouches that are included with the viscoelastic syringe.
UNITS: 3,720 California and North Carolina
-----------
COMPANY: Beckman Coulter, Inc.
PRODUCT: Cyto-Stat/Coulter Cone B6-RD
REASON: Diminished expression on B-cell population when drawn in EDTA tubes, which may lead to inaccurate interpretation of phenotype results
UNITS: 1053 Nationwide and Canada
The FDA has issued numerous warning letters to PointCare Technologies, a leading developer and producer of flow cytometry products. In its latest warning letter dated 14 June 2011, the FDA cited PointCare’s repeated and unresolved problems with their testing equipment, reagents and manufacturing facilities:
“…two of these three lots failed the specifications for osmolality and optical density (OD)… devices are adulterated… their manufacture, packing, storage, or installation are not in conformity with the Current Good Manufacturing Practice (CGMP) requirements… did not perform adequate stability studies after changing the packaging of CD4NOW Gold Reagent to determine an accurate shelf-life for the product… functional performance of the gold pack was not acceptable… failed to provide scientific justification for performing gold functional testing on only one vial per lot… Failure to establish, maintain, and implement a corrective and preventative action procedure… due to an AC charging cable/adapter bursting into flames while service personnel charged the unit… Failure to have quality audits conducted by an individual that does not have direct responsibility over the matters being audited…” (emphasis added)THE MARKETING OF JUNK SCIENCE
Of the 66 aforementioned recalls and warning letters, FDA complaints were issued for failures that typically result in low CD4 counts. For agencies that need low counts to claim high HIV infection rates e.g. revenues (and for clinicians who accept kickbacks and bribes from companies like Bristol-Myers Squibb [BMS] and Gilead Sciences), flow cytometry helps clinicians justify the unnecessary delivery of toxic HAART therapies to healthy patients.
Flow cytometry is especially helpful in places like Africa, where mining companies routinely dump toxins and heavy metals into waterways.
One company is Kilembe Mines Limited, which is trying to sell an operation that has polluted the environment and water supplies of southwestern Uganda since 1956. Because foreign investors are reluctant to assume liability that comes with the purchase of toxic waste dumps, clinics and medical devices that blame pollution-caused ailments on HIV offer significant advantages to prospective investors.
According to this report, the Uganda Catholic Medical Bureau (UCMB) has distributed drugs in this area for 30 years.
In an effort to “scale up access to antiretroviral therapy…” UCMB advised that “treatment procedures and the monitoring of clients are simplified so that lower cadres of health workers can be trained to carry out some of the simpler functions… (of HIV testing, diagnosis and treatment).”
Gloria Kakuru of the Baylor University’s International Pediatric AIDS Initiative (BIPAI) explains how PointCare Now helps these “clinicians” diagnose HIV in the area surrounding the Kilembe mines – claiming that the device is superior because it justifies earlier initiation of HAART medication in adults and children:
These inflated misdiagnoses are then used by agencies like UNAIDS, the World Health Organization (WHO) and drug companies like Bristol-Myers Squibb (BMS), GlaxoSmithKline, Merck (and others) to push deadly HIV drugs – usually at taxpayer expense.
Although labs continue to use PointCare Now and its CD4 Gold reagents, the recent FDA warning letters do not appear on the PointCare website or in any of these marketing materials – nor is there any evidence that PointCare, clinical laboratories or advertisers made any attempt to contact patients who are misdiagnosed by the faulty equipment and protocols, poisoned by HAART medications or criminally convicted for having sex.
RELIABILITY
Dr. Marion Joppe describes “The Research Process” this way:
“The extent to which results are consistent over time and an accurate representation of the total population under study is referred to as reliability. In other words, if the results of a study can be reproduced under a similar methodology, then the research instrument is considered to be reliable.”Theoretically, reliable tests should deliver the same results no matter how many times it is applied to random members of the same target groups.
Because of the failure to conduct population studies that would have helped researchers understand CD4+ variability with age, sex, race, time of day or health status; along with the lack of standardization, the reliability of the HIV test results of the absolute CD4+ T cell value by flow cytometry is, at best, wholly unreliable.
While caution must be exercised in the interpretation of unreliable results, this has not been emphasized by the CDC. White blood cell counts can vary substantially from day to day and may account for shifts of as much as 50 to 150 in normal adults, although the degree of this change may be less in individuals with lower CD4 counts. Also substantial variability exists from laboratory to laboratory; those that do not perform cell count procedures frequently – or do not have quality assurance programs – can be expected to produce inaccurate test results. Compounding this problem, an extended delay of more than 48 hours between the time of sampling and actual specimen processing will result in inaccurate values. Therefore, if a laboratory does not perform the test on a daily basis, or if, for example, blood is drawn on Friday and processed on Monday, the test results may be inaccurate. Another common source of inaccuracy is refrigeration of the blood sample.
Because caution conflicts with their ongoing social marketing campaigns, the CDC ignores the known science of CD4+T cell subsets, the Th1/Th2 dual strategy of the immune system, the function of nitric oxide in cell mediated immunity and the possibility for reversal of this immune imbalance with inexpensive nutracuticals, antioxidants and detoxification.
During the two years of its involvement in HIV-related criminal cases and its review of medical and laboratory records, OMSJ has found no evidence that US laboratory facilities exercise caution in the use of flow cytometry equipment or have met any of the CDC’s recommended standards. Instead, laboratories shroud their operations in secrecy, which is only exposed when laboratories like Quest Diagnostics pay $241 million in fines for fraud and kickbacks or $302 million for illegally marketing misbranded diagnostic equipment.
Given these findings, there is no credible evidence that HIV is responsible for the decrease in CD4+T cells in acquired immune deficiency or that a decrease in CD4+T cells is a unique finding in people who are HIV+: Nor is there any credible evidence that the current usage of flow cytometry technology is justified as a diagnostic therapeutic tool in the current HIV/AIDS paradigm. Unfortunately, it is unlikely that clinicians will abandon these voodoo technologies anytime soon.
Dr. Nancy Banks is a graduate of Hunter College and Harvard Medical School. She practiced general obstetrics and gynecology for 25 years and is an expert in the testing, diagnosis, and treatment of sexually-transmitted diseases. She is the author of the award-winning book AIDS, Opium, Diamonds & Empire (2010). Clark Baker is OMSJ’s Principal Investigator.
.
- KCQuestor
- 05-04-2012, 01:12 PM
Dude, just post the link. You don't need to send the full text. Unless of course you are just trying to shovel shit to cover up the fact that you are overflowing from both ends. Nah, you couldn't be doing that. The Baker/Banks article that you posted in its fucking entirety has been debunked numerous times. It is all balderdash.
http://hivinnocenceprojecttruth.com/...ometry-part-i/
http://hivinnocenceprojecttruth.com/...low-cytometry/
If you had just posted the link, we could have told you that without you filling four useless posts of nonsense.
Stacy, I saw your links, and they were very informative. I just wanted to respond to his silly request that we show him a paper that says "HIV causes AIDS". No one questions that the measles virus causes measles or the ebola virus causes ebola. Maybe this whole conspiracy would never have been born if we had named the virus after the syndrome. No sane person would ask if the AIDS virus cased AIDS
http://hivinnocenceprojecttruth.com/...ometry-part-i/
http://hivinnocenceprojecttruth.com/...low-cytometry/
If you had just posted the link, we could have told you that without you filling four useless posts of nonsense.
Stacy, I saw your links, and they were very informative. I just wanted to respond to his silly request that we show him a paper that says "HIV causes AIDS". No one questions that the measles virus causes measles or the ebola virus causes ebola. Maybe this whole conspiracy would never have been born if we had named the virus after the syndrome. No sane person would ask if the AIDS virus cased AIDS

- Mr. Bill
- 05-04-2012, 08:55 PM
Evidence of HIV fraud
A Selection of HIV Documents
Unearthed by US Governmental Investigations into the scientific work of Dr. Robert Gallo
As made available in "Fear of the Invisible" by Janine Roberts
A Selection of HIV Documents
Unearthed by US Governmental Investigations into the scientific work of Dr. Robert Gallo
As made available in "Fear of the Invisible" by Janine Roberts
For Commentary on these papers, please see the accompanying article "How the HIV Papers were Fixed"
A. Letter from Dr. Gonda XE "Gonda, Matthew" , the Head of Electron Microscopy at the NIH XE "National Institutes of Health" , to Popovic XE "Popovic, Mikulas" , copied to Gallo XE "Gallo, Robert" . He reports that images wanted for the Science papers, do not contain HIV XE "HIV" (HTLVIII) a s Gallo had claimed, but only cellular rubbish. This was received only 3 days before Gallo sent in the Science papers for publication, When the papers appeared in print, they still contained photos credited to Gonda, with Gallo saying they contain HIV.
see:
http://www.fearoftheinvisible.com/im...c_03.26.84.jpg
B. The first and the most important of the four Science Papers said to prove HIV XE "HIV" the cause of AIDS XE "AIDS: Autoimmune Deficiency Syndrome" . This is the typed draft produced by the Lead Author M. Popovic XE "Popovic, Mikulas" , with all the handwritten editing and comments made by R. Gallo XE "Gallo, Robert" just 7 days before the manuscript went in for publication. (The cover page unfortunately has faded.)
see:
http://www.fearoftheinvisible.com/im...ed_page_01.jpg
http://www.fearoftheinvisible.com/im...ed_page_02.jpg
http://www.fearoftheinvisible.com/im...ed_page_03.jpg
http://www.fearoftheinvisible.com/im...ed_page_04.jpg
http://www.fearoftheinvisible.com/im...ed_page_05.jpg
http://www.fearoftheinvisible.com/im...ed_page_06.jpg
http://www.fearoftheinvisible.com/im...ed_page_07.jpg
http://www.fearoftheinvisible.com/im...ed_page_08.jpg
http://www.fearoftheinvisible.com/im...ed_page_09.jpg
End of Popovic's Draft.
Extra pages of Gallo's notes attached to draft
see:
http://www.fearoftheinvisible.com/im...ed_page_10.jpg
http://www.fearoftheinvisible.com/im...ed_page_11.jpg
http://www.fearoftheinvisible.com/im...ed_page_12.jpg
http://www.fearoftheinvisible.com/im...ed_page_13.jpg
http://www.fearoftheinvisible.com/im...ed_page_14.jpg
http://www.fearoftheinvisible.com/im...ed_page_15.jpg
C. In this letter Gallo explains why HIV (here called HTLV) is ‘extremely rare' in the AIDS patients. This is dated 1 day before he sent his papers claiming HIV causes AIDS for publication in the Science journal
see:
http://www.fearoftheinvisible.com/im...a_03.29.84.jpg
source: http://www.fearoftheinvisible.com/fr...rch-background
.
- Mr. Bill
- 05-04-2012, 09:02 PM
Robert Gallo Defends Himself
See expose of fraud and Robert Gallo's letter:
http://www.fearoftheinvisible.com/gallodefendsemai
Written by Janine Roberts
I was astonished by what Gallo had chosen to object to. He did not object to what I wrote about the last minute changes he made to the Popovic paper, nor did he object to my citations from the devastating conclusions of the ORI or from the Inspector General's investigations into his work, but only to my mention of what the Secret Service had discovered in his papers.
After receiving this, I thought it best to check what the Secret Service had to say, to make sure I had not made any errors. I have previously investigated many intelligence operations. This has given me contacts I can call on when needed. Within three days, I had the former Head of the Secret Service on the phone, Larry Stewart, the man who had led their investigation into Robert Gallo's HIV research.
He confirmed that they found convincing evidence that many of Gallo's laboratory documents were ‘fixed' prior to being presented as evidence `- and were thus fraudulent.
But I was not the first to cite these Secret Service findings. They are also presented in some detail in John Crewdson's 2003 book, Science Fictions, as Gallo surely must have known. If he had solid legal objections to my brief mention of the Secret Service findings, then I am sure he would have taken it up earlier with Crewdson.
Crewdson detailed how the Secret Service's forensic laboratory had proved that laboratory records presented as evidence by Gallo were fraudulently created, not on the date stated, not when the experiment was done, but later. This was particularly obvious in one document dated 1984 that reported the use of ‘HIV' - years before the virus was given this name.
continued in detail: http://www.fearoftheinvisible.com/gallodefendsemai
.
See expose of fraud and Robert Gallo's letter:
http://www.fearoftheinvisible.com/gallodefendsemai
Written by Janine Roberts
I was astonished by what Gallo had chosen to object to. He did not object to what I wrote about the last minute changes he made to the Popovic paper, nor did he object to my citations from the devastating conclusions of the ORI or from the Inspector General's investigations into his work, but only to my mention of what the Secret Service had discovered in his papers.
After receiving this, I thought it best to check what the Secret Service had to say, to make sure I had not made any errors. I have previously investigated many intelligence operations. This has given me contacts I can call on when needed. Within three days, I had the former Head of the Secret Service on the phone, Larry Stewart, the man who had led their investigation into Robert Gallo's HIV research.
He confirmed that they found convincing evidence that many of Gallo's laboratory documents were ‘fixed' prior to being presented as evidence `- and were thus fraudulent.
But I was not the first to cite these Secret Service findings. They are also presented in some detail in John Crewdson's 2003 book, Science Fictions, as Gallo surely must have known. If he had solid legal objections to my brief mention of the Secret Service findings, then I am sure he would have taken it up earlier with Crewdson.
Crewdson detailed how the Secret Service's forensic laboratory had proved that laboratory records presented as evidence by Gallo were fraudulently created, not on the date stated, not when the experiment was done, but later. This was particularly obvious in one document dated 1984 that reported the use of ‘HIV' - years before the virus was given this name.
continued in detail: http://www.fearoftheinvisible.com/gallodefendsemai
.
- Guest030817
- 05-04-2012, 09:13 PM
Fantastic! Let's encourage people to not get tested.
I don't care what your conspiracy theories tell you. Bacteria and virus' are clearly distinguished from one another under a microscope. Anyone who has taken microbiology has been down this road and understands. General population at large...not so much. I do a complete panal testing every 6 weeks max and have a private consultation with the epidemiologist.
I don't care what your conspiracy theories tell you. Bacteria and virus' are clearly distinguished from one another under a microscope. Anyone who has taken microbiology has been down this road and understands. General population at large...not so much. I do a complete panal testing every 6 weeks max and have a private consultation with the epidemiologist.
- Mr. Bill
- 05-04-2012, 09:31 PM
Fantastic! Let's encourage people to not get tested. Originally Posted by Ari816I thoroughly agree!

The AIDS Trap printable document
Download the PDF, print and distribute widely!

Available in 7 languages.
.
- Mr. Bill
- 05-04-2012, 09:45 PM
Guys, as soon as he cited (I said "cited", not "quoted", you illiterate idiot) Duesberg the game was up. If ever there was a discredited "expert", it is him.Yes a tiny oversight on my behalf, however, if you were so concerned with correctness and being right, you'd have the integrity and balls to investigate the massive fraud that surrounds the HIV/AIDS hypothesis.
Dammit Jackie and SillyGirl. I'm back in it. Fuck me. Originally Posted by KCQuestor
And as for Peter Duesburg, he's one of the most credentialed and respected researchers on the planet. As only a simple-minded anonymous poster on a SHMB, your criticism of Professor Duesburg's accomplishments amounts to squat.
http://www.virusmyth.com/aids/data2/pdbio.htm
.